Playlist
Show Playlist
Hide Playlist
Action of Transamidase – Beta Lactam Antibiotics
-
Slides 14 Chemistry Advanced Le Gresley.pdf
-
Download Lecture Overview
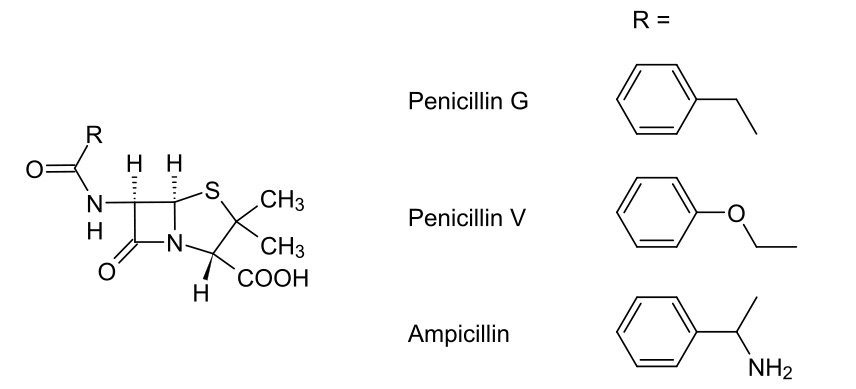
00:00 Here we have a penicillin-binding protein shown as a green ovoid there with PBP, the abbreviation for the penicillin-binding protein. I’ve also shown that it’s got CH2OH. This correlates to the serine amino acid which is at its active site. 00:20 Now, if you recall, we said that the terminus which is activated of the polypeptide contained two alanine groups: D-Alanine-D-Alanine. I’ve shown those here. I’ve also shown them attached to the peptide chain, but I haven’t shown the rest of the sugar because there really isn’t enough space. The way in which penicillin-binding protein works is it attacks the carbonyl-carbon of that amide and it forms an ester group with the second amine... second alanine on that polypeptide and kicks off the alanine, as you can see here on the right hand side. 01:02 Look at what we’ve done. We’ve converted an amide into an ester. And do you remember how we were talking back in Module III, how that’s a very difficult to achieve from a synthetic perspective? This is the advantage in this case of the enzymatic activity for this biological process because it is able to achieve this. It converts it into an ester via a covalent bond formed and the terminal, the alanine, diffuses away. 01:28 What then happens is that the neighbouring pentaglycyl unit attached to the other polysaccharide chain then comes in and attacks that carbonyl-carbon via an addition-elimination reaction. What then happens is the formation of an amide and the diffusing away of the penicillin-binding protein as a reactivated enzyme to begin again. 01:56 Pay particular attention to the bottom right hand corner where I show what we’ve done is we’ve linked a polysaccharide to one peptide chain to, then, the peptide chain with the other polysaccharide. We have cross-linked the polysaccharide chains using these polypeptide bridges. Once the enzyme is restored, it can carry on and do its thing. 02:20 Penicillins, though, work to inhibit this process. And they’re thought to resemble closely the geometry of an acylated D-Alanine-D-Alanine dipeptide. As you can see here on the right hand side, penicillin or the general structure for it, is shown to the middle on the right hand side and at the bottom right hand corner, an acylated D-Alanine-D-Alanine is shown. 02:42 As you can see, the stereochemical orientation is remarkably similar and this forms a key part of it as a pharmacophore for inhibiting the penicillin-binding protein. 02:53 Transamidase or penicillin-binding protein mistakes it for its normal substrate and as we’ll see in a second, this results in the irreversible inhibition of the penicillin-binding protein and prevents the crosslinking of the peptidoglycan. Because of the ring system, the hydrolysis does not result in penicillin breaking into two units like a D-Alanine-D-Alanine dipeptide would. And the heterocyclic ring, this thiazolidine ring, is thought to be a steric barrier to the approach of the pentaglycyl unit. As a consequence of this, the enzyme is irreversibly inhibited and cross-links cannot form. Resulting in this, is that the cell wall becomes structurally weak, leading to cell lysis and death. Because, bear in mind, the bacteria is under constant attack from the host’s own immune system. 03:45 So, let’s have a look at that in a bit more detail, shall we? So, here we’ve got the diagram I showed you before. We’ve got the D-Alanine-D-Alanine cell-wall fragment. We’ve got the serine hydroxyl and the active site of the penicillin-binding protein. What happens in this scenario is that we get attack by the serine hydroxyl group, as we showed before, which cleaves in between the D-Alanine-D-Alanine dipeptide, converting it into an ester and the terminal D-Alanine diffuses away. 04:16 Under normal circumstances, a pentaglycyl unit attached to the neighbouring polysaccharide and peptide chain would enter the active site and attacks the enzyme peptidoglycan complex, thus resulting in amide-bond formation and regeneration of our penicillin-binding protein. 04:33 However, in the presence of penicillin what happens is this. The serine hydroxyl, mistaking the penicillin for its natural substrate, attacks the beta-lactam group at the carbonyl-carbon, thus opening up that carbon-oxygen double bond and breaking open the nitrogen-carbon double bond. As you can see here in this scenario in the bottom left hand corner, we have now generated a penicillin-binding protein which has been esterified by the penicillin, forming an ester bond here. Now, of course, you might think well it’s an ester, surely that could hydrolyse. But, that’s where the thiazolidine ring comes in, ostensibly blocking the active site and preventing other molecules from entering. This enzyme is now inactive and has to be broken down and reformed by the bacteria. 05:25 So, that’s brilliant. But, there are problems.
About the Lecture
The lecture Action of Transamidase – Beta Lactam Antibiotics by Adam Le Gresley, PhD is from the course Medical Chemistry.
Included Quiz Questions
The active site of penicillin-binding protein (PBP) contains which of the following?
- Serine residue
- Alanine residue
- Tryptophan residue
- Glycine residue
- Methionine residue
Which of the following statements about peptidoglycan formation mechanism is NOT true?
- During the cross-link formation, the penicillin-binding proteins get incorporated into the bridge structure.
- The penicillin-binding protein attacks the carbonyl carbon of the amide group between terminal D-ala-D-ala units.
- After the formation of a cross-link between two peptide chains, the penicillin binding protein gets free to start new cross-link formation.
- After the removal of D-alanine unit, the neighboring pentaglycine unit from the other NAM-NAG polysaccharide chain attacks the carbonyl carbon via an addition-elimination reaction.
- The PBP forms an ester group with the second alanine residue of the peptide chain and kicks off the first D-alanine unit from peptide chain.
The basis of irreversible inhibition of penicillin-binding protein via penicillin is which of the following?
- The geometrical resemblance of penicillin molecule to an acylated D-alanine-D-alanine dipeptide.
- The geometrical resemblance of penicillin molecule to pentaglycine chain.
- The geometrical resemblance of penicillin to serine residue present in the active site of PBP.
- The geometrical resemblance of penicillin to NAM units.
- The geometrical resemblance of penicillin to NAG units.
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
5 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |