Playlist
Show Playlist
Hide Playlist
Ammonia: NH₃ & NH₄
-
Slides 08 MetabolicAcidosisAlkalosis AcidBaseBalance GeneralPhysiology.pdf
-
Download Lecture Overview
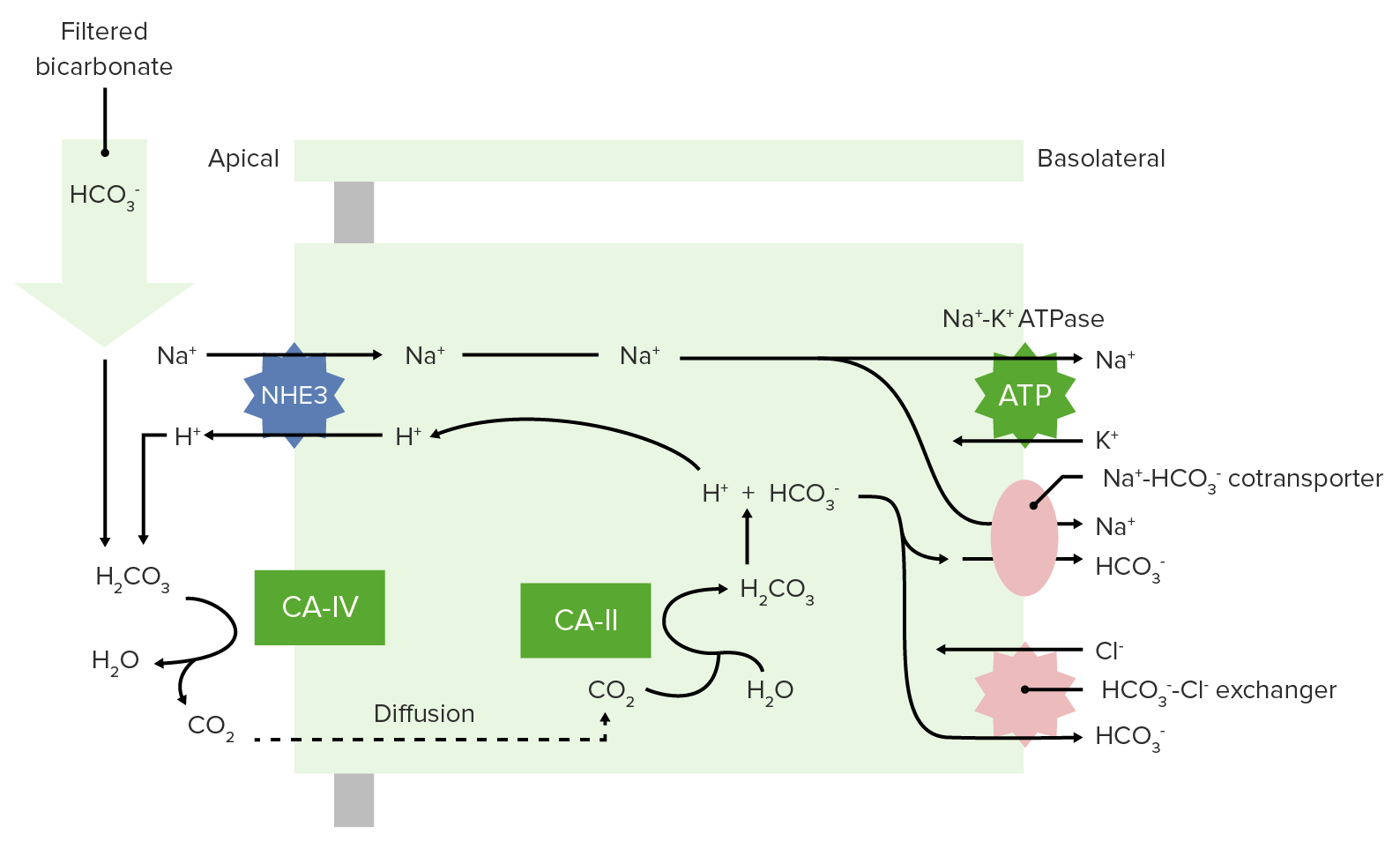
00:01 Okay, so we’ve dealt with about 40 percent of our non- volatile acids. 00:08 That still leaves 60 percent if you’re a math wiz. 00:13 So, what do you do with here? You have to have something else be a part of the process. 00:19 In this case, we have NH3 and NH4 that’s going to take care of the rest of this non- volatile acids. 00:27 So, this ammonia, ammonium response is very important. 00:32 You utilize the same kinds of mechanisms the sodium hydrogen ion exchanger and what we’ll do is you’ll pump some of the NH4 out through this exchanger. 00:48 Where does that come from? It comes from metabolism. The NH4 comes from metabolism. 00:55 A little bit of NH3 is formed as part of this reaction involving alpha-ketoglutarate. 01:04 NH3 is not charged enough and will be able to move through the membrane. 01:10 NH4 has a charged and therefore has to be exchanged through this transporter. 01:17 So, whether you use NH4, NH3 it’s not important, that’s just how you’re getting the molecule out across the apical membrane. 01:27 You noticed when you have made an NH4 or NH3, a new bicarbonate is also formed. 01:35 And that gets pushed across the basolateral membrane and reabsorbed by the blood. 01:41 Therefore, you have NH4 of being your excreted acid. 01:47 So, it doesn’t matter how you get it there, even the NH3 by itself bind with the hydrogen ion or kicked out an NH 4. 01:54 You will lose it as NH4. 01:58 This involves about 60 percent of your non- volatile acids will be removed in this manner. 02:05 It’s all done in the proximal tubule. 02:08 The other important thing about NH3/NH4 is that it is highly adaptable. 02:16 If you have a chronic academia condition, this NH3/NH4 mechanism up regulates. 02:25 You Titratable acids don’t, they pretty much stay the way they are. 02:29 But this ability to increase the amount of hydrogen ion you can kick out across the apical membrane through NH3 or NH4 can increase to help you adopt to that environment. 02:43 So, when you think about the amount of millimoles that we started off with, with let’s say 70 millimoles of non- volatile acids. 02:52 About 30 millimoles of that were taking care of through our Titratable acids in the proximal tubule, the distal convoluted tubule and the collecting duct. 03:04 About 15 millimoles in a proximal tubule, about 5 millimoles in the distal convoluted tubules and about 10 millimoles in the collecting duct. 03:13 We’re taking care of our Titratable acids. 03:17 The other 40 millimoles we’re taking care by NH3/NH4.
About the Lecture
The lecture Ammonia: NH₃ & NH₄ by Thad Wilson, PhD is from the course Acid-Base Balance.
Included Quiz Questions
In which part of the kidney do H+ ions get excreted using NH3 / NH4?
- Proximal convoluted tubule
- Distal convoluted tubule
- Collecting duct
- Glomerulus
- Bowman's space
Which of the following molecules is generated for every NH3/NH4 molecule that is formed?
- HCO3
- Phosphate
- Pyruvate
- Lactate
- Urate
Which of the following statements is FALSE regarding the NH3/NH4 mechanism of excretion of H+ ions?
- The titratable acid mechanism can adapt by upregulating increased excretion of non-volatile acids.
- NH3 /NH4 mechanism can adapt by upregulating increased excretion of non-volatile acids.
- About 60% of the non-volatile acids are eliminated using NH3/NH4.
- NH4 is the form in which the non-volatile acid is eliminated.
- NH3 is able to cross the plasma membrane.
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
5 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |