0th and 1st Law: Temperature and Energy
About the Lecture
The lecture 0th and 1st Law: Temperature and Energy by Jared Rovny, PhD is from the course Thermodynamics and Thermochemistry.
Included Quiz Questions
Which of the following statements is FALSE?
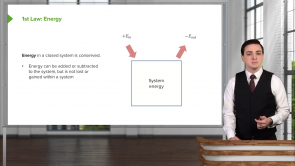
- Energy can neither be added nor taken from a system but it can be lost or gained within a system
- Temperature, pressure and volume are examples of state variables
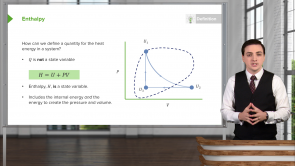
- State variables are the quantities that only depend on the current state
- If two objects are in thermal equilibrium with the third, they are all in equilibrium with each other
- Energy in a closed system is conserved
These courses may be of interest to you
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
1 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |
So cool!
By Tatiana B. on 04. January 2022 for 0th and 1st Law: Temperature and Energy
man, i was so confused about this topic but now I'm clear , I'm very pleased
Quiz Overview
wrong
right
open