Playlist
Show Playlist
Hide Playlist
Targeted Protein Degradation
-
Slides Cellular Pathology - Degradation.pdf
-
Download Lecture Overview
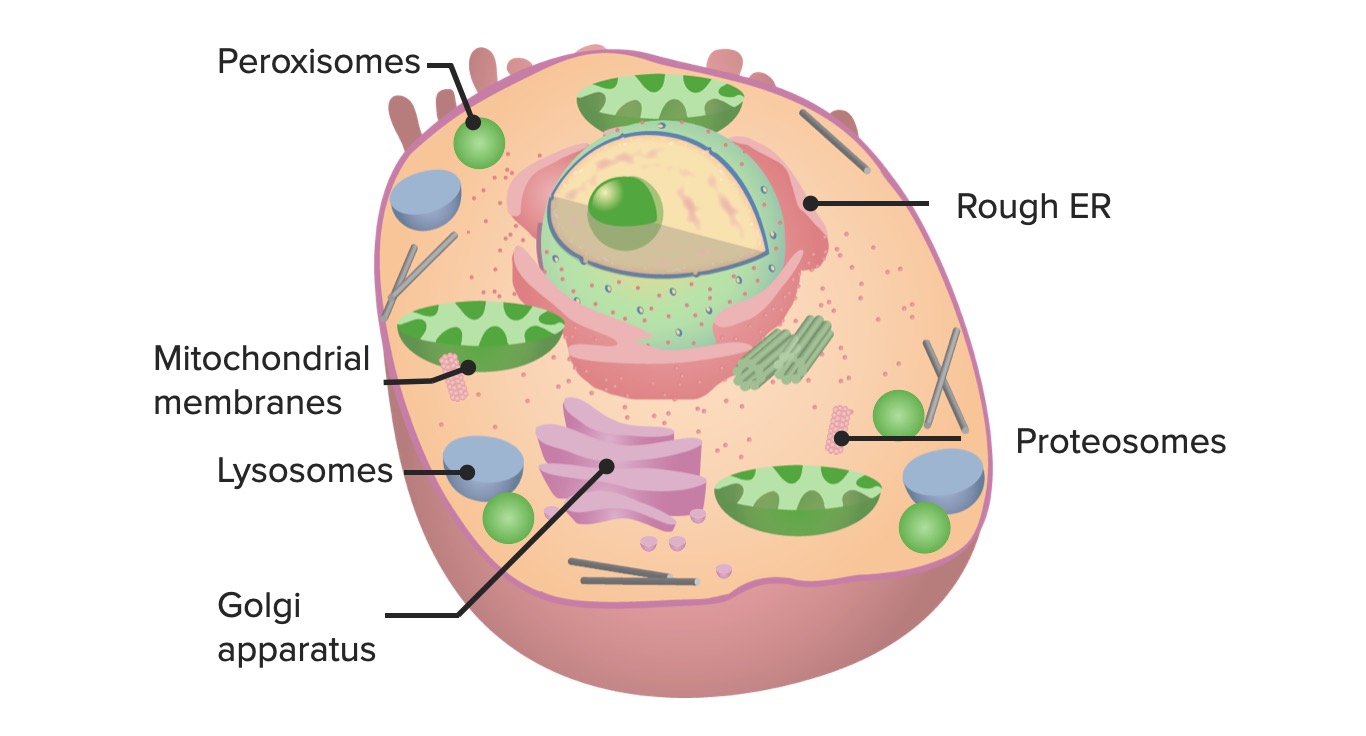
00:01 All right, so the next topic we're going to discuss is degradation. 00:06 We've talked about synthesis. On the other side, we have to break things down. 00:10 Stuff that's taken up from the outside world, that's obvious, but we also have to break down senescent organelles or proteins that we no longer need or that have accumulated mutation. 00:22 So, degradation is an important flipside of a coin that we have talked about in previous topic discussions. 00:28 All right, so here's where we are on the roadmap of basic cell housekeeping functions. 00:34 We're at item number six, degradation. 00:37 All right, let's talk about targeting little things for degradation first, talking about proteins. 00:44 How do we say, "You. You protein right there, you need to be degraded." Well, it's actually a remarkably complex series of steps that are important to understand, because increasingly, we use these as therapeutic targets. 01:00 So, don't just think you're being hammered on the details here. 01:04 They, in fact, are going to be really useful in thinking about therapies for certain diseases. 01:10 Okay, so let's start. We haven't yet even identified which protein we're going to target, but even before we do that, we load up a number of proteins. 01:19 These are called E ligases, and they're labeled E1, E2, and E3. Clever names. 01:26 Those ligases are going to be important for delivering this molecule at the very top, ubiquitin. 01:34 Ubiquitin, just like its name indicates, is ubiquitous. It's in every nucleated cell. 01:41 And it's gonna be responsible for ultimately targeting proteins for degradation. 01:46 So, you have your first E1 ligase, and it then interacts with ubiquitin and some ATP, and loads it up, so now we have a ubiquitin attached to the E1 ligase. 02:00 Just the first step. Again, the chemistry, not too important, but understanding the multiple stages that we're gonna go through. 02:08 That E1 ligase with that ubiquitin attached is now gonna transfer it to the E2. 02:16 So, it's -- it is never simple in biology, because we're overengineered, but this is the intermediate step to E2. 02:24 And then, finally, E2 is going to transfer that ubiquitin in combination with E3 to our target protein. 02:33 So, you see that squiggle down there attached or adjacent to the E3? That's gonna be our target protein. 02:39 And the E2-E3 complex is gonna take the ubiquitin that's sitting there and attach it to our target. 02:48 And it's gonna say, "You, you're gonna be degraded." That now is our signal, bit it -- one, ubiquitin is never enough. 02:55 You always have to have a whole bunch. 02:57 So, you have to go through this process multiple, multiple times until we get a protein multiply tagged with long chains of ubiquitin. 03:05 It seems like a lot of work and a lot of effort to just say, "This protein needs to go away," but we want to be very careful in how we target proteins for degradation, cuz we might need them, or we don't wanna degrade them inappropriately. 03:20 So, this is carefully regulated. And why do we care? Because a lot of these ligases are therapeutic targets. 03:26 Okay, so now we've targeted this protein. 03:29 We say, for some reason, this protein needs to go away. 03:32 It's senescent, it's misfolded, we no longer need it. Now, what? Okay, so here, we have the protein, we've put on our ubiquitin tag, and now, there's this intracellular set of protein complexes that form basically an intracellular little, tiny garbage disposal. 03:51 There are multiple subunits in this. 03:53 It forms a core. It really kind of looks like that. 03:56 And we take the targeted protein with a ubiquitin on, and the ubiquitin says, "This needs to go to the proteasome," and it basically tosses it into the garbage disposal, and we come out with little peptide fragments at the other end. 04:11 We have degraded that protein. 04:13 In the process, the ubiquitin is released, and we recycle it. 04:17 So, we use it over and over and over again. 04:19 But that's the process of how we degrade little things into little peptide fragments after we've targeted them with ubiquitin. 04:27 So, what kind of processes do these proteasomes, these little miniature garbage disposals play? They're very important in cell cycle. 04:35 So, if we tell a cell to go through cell cycle and we have a cyclin and a CDK kinase that has been upregulated, we don't want that to stay upregulated for a long period of time, so we degrade them in a very coordinated fashion. 04:47 So, cell cycle critically depends on the proteasomes to break down things when we don't need them anymore. It's important in transcription. 04:55 So, transcription factors, we don't want them to be active and active and active over a period of time. 05:00 We want them to go away cuz we're done transcribing. 05:03 Proteasomes play that role. It's in quality control. 05:06 If we have a misfolded protein, we attach ubiquitins to it and we get it gone. 05:12 So, it's important for quality control. 05:14 It's important for DNA repair. 05:16 So, understanding, organizing, and recognizing when there's damage. 05:19 And coordinating the repair is also a job also involving proteasomes. 05:25 The stress response? In a previous topic, we covered the endoplasmic reticulum stress response that occurs when we have too many misfolded proteins, and clearly, proteasomes are involved in that. And in signal transduction. 05:40 Again, once we have a ligand bind to its receptor and activate downstream molecules, we don't want those molecules to continue to signal. 05:49 We want it to one-time signal, be done, and go away. 05:52 Proteasomes are responsible for that. 05:55 And immunity. It turns out, the proteasomes are the way by which we surveil the intracellular environment, looking for pathogens that live inside the cell, and break it into little fragments that we can then use the immune system to recognize. 06:10 And we'll come back to that in a subsequent topic discussion. 06:13 So, proteasomes, really important, and you may not have even recognized or realized that they were there.
About the Lecture
The lecture Targeted Protein Degradation by Richard Mitchell, MD, PhD is from the course Cellular Housekeeping Functions.
Included Quiz Questions
Which of the following is responsible for targeting proteins for degradation?
- Ubiquitin
- Caspase
- Heat shock protein
- Endonuclease
- Clathrin
Proteasomes play a major role in...?
- ...regulating the cell cycle.
- ...cell trafficking.
- ...upregulating signal transduction.
- ...metabolizing long-chain fatty acids.
- ...synthesizing secretory proteins.
What is the role of ligases in the protein degradation process?
- They attach ubiquitin to damaged or misfolded proteins.
- They act as proton pumps to facilitate the proteolytic process.
- They are involved in refolding damaged or misfolded proteins.
- They degrade ubiquitin after the protein degradation process is finished.
- They directly activate lytic enzymes in proteosomes.
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
5 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |