Nuclear Properties
About the Lecture
The lecture Nuclear Properties by Jared Rovny, PhD is from the course Atomic Nucleus.
Included Quiz Questions
Which of the following choices represent nucleons?
- Protons, neutrons
- Protons, neutrons, electrons
- Neutrons, electrons
- Neutrons, photons
- Photons, protons, neutrons, electrons
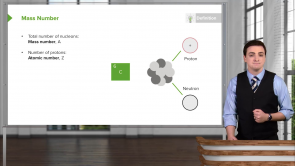
Nitrogen has an atomic number of 7. How many protons and neutrons are in the nucleus of ¹⁵N?
- 7 Protons, 8 neutrons
- 8 Protons, 7 neutrons
- 15 Protons, 7 neutrons
- 7 Protons, 15 neutrons
- 7 Protons, 1 neutron
Which of the following choices could be an isotope of carbon?
- Mass number 14, atomic number 6, neutron number 8
- Mass number 28, atomic number 14, neutron number 14
- Mass number 11, atomic number 5, neutron number 6
- Mass number 13, atomic number 7, neutron number 6
- Mass number 10, atomic number 4, neutron number 6
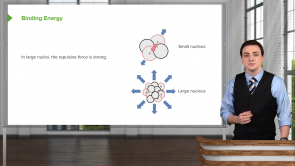
If carbon has an atomic number of 6, how can its atomic weight be 12.011 g/mol?
- The weight depends on the number of nucleons, which is usually 12 and rarely 13 in natural abundance.
- The weight is always twice the atomic number, plus a contribution from the binding energy of the atoms.
- The weight is the weight of the most common isotope found in nature, which has a weight a bit more than 12 g/mol.
- In reality, physical quantities will never be exactly the theoretical values.
- Carbon always appears in nature as a molecule of 2 carbon atoms hence the atomic weight is double the atomic number.
These courses may be of interest to you
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
5 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |
Quiz Overview
wrong
right
open