Bohr Model and Atomic Structure
About the Lecture
The lecture Bohr Model and Atomic Structure by Jared Rovny, PhD is from the course Electronic Structure.
Included Quiz Questions
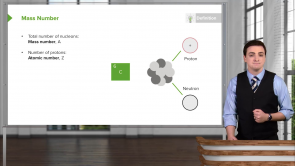
Before the Bohr model, how were positive charges considered to be distributed in an atom?
- Spread out throughout the atom
- Concentrated in the center
- Orbiting the center
- Forming concentric rings
- Spread uniformly in a spherical shell
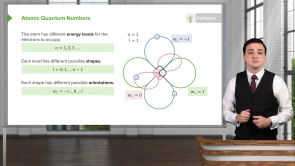
What are the possible energy levels n of an electron in an atom? And what is the maximum number of electrons that can occupy each level?
- n = 1, 2, 3, ..., Max. number of electrons at each level : 2n²
- n = 0, 1, 2, ..., Max. number of electrons at each level : n²
- n = -2, -1, 0, 1, 2, ..., Max. number of electrons at each level : 2n²
- n = 1/2, 2/2, 3/2, ..., Max. number of electrons at each level : n²
- n = 0, 1/2, 2/2, 3/2, ..., Max. number of electrons at each level : 2n²
How many electrons can occupy the n = 3 energy level?
- 18
- 16
- 20
- 14
- 10
How is a photon emitted from an excited atom? And what is the relation between the emitted photon energy E and its frequency f?
- When electrons in an atom are dropped from an excited state to a lower energy state the energy difference is released in the form of the emission of a photon, E = hf
- By raising electrons to higher energy levels, E = hf
- By swapping two electrons in adjacent energy levels, E = E₀ f/f₀
- By losing an electron from the atom, E = E₀ f/f₀
- The excited atom collides with other atoms and releases its kinetic energy in the form of an emitted photon, E = hf
These courses may be of interest to you
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
5 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |