Playlist
Show Playlist
Hide Playlist
Approach to Acid Base Status: Step 3 – Laboratory Diagnostics
-
Slides DiagnosticsAcidosisAlkidosisStep3-5 RespiratoryPathology.pdf
-
Download Lecture Overview
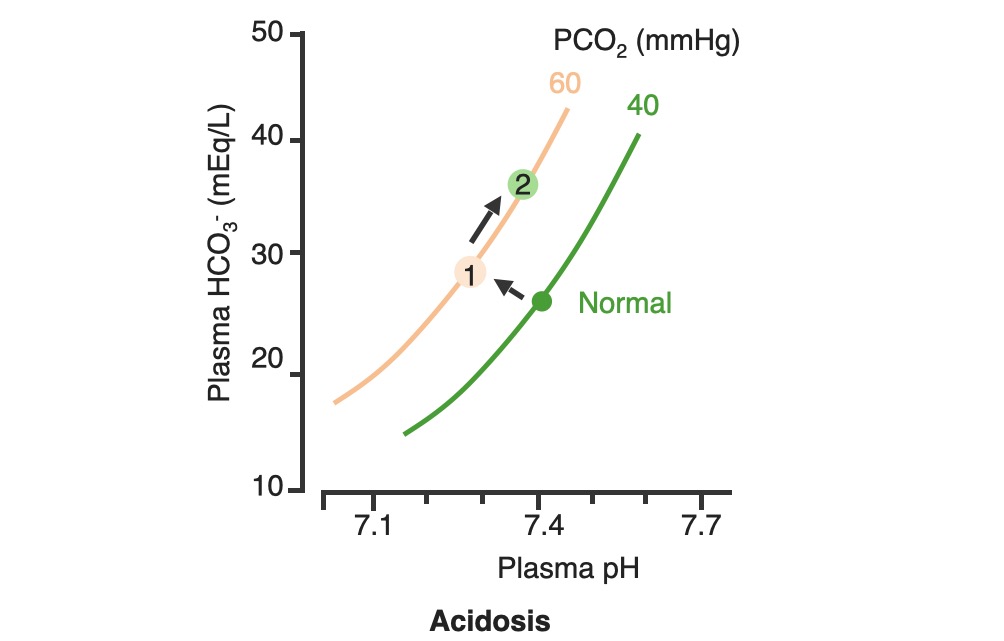
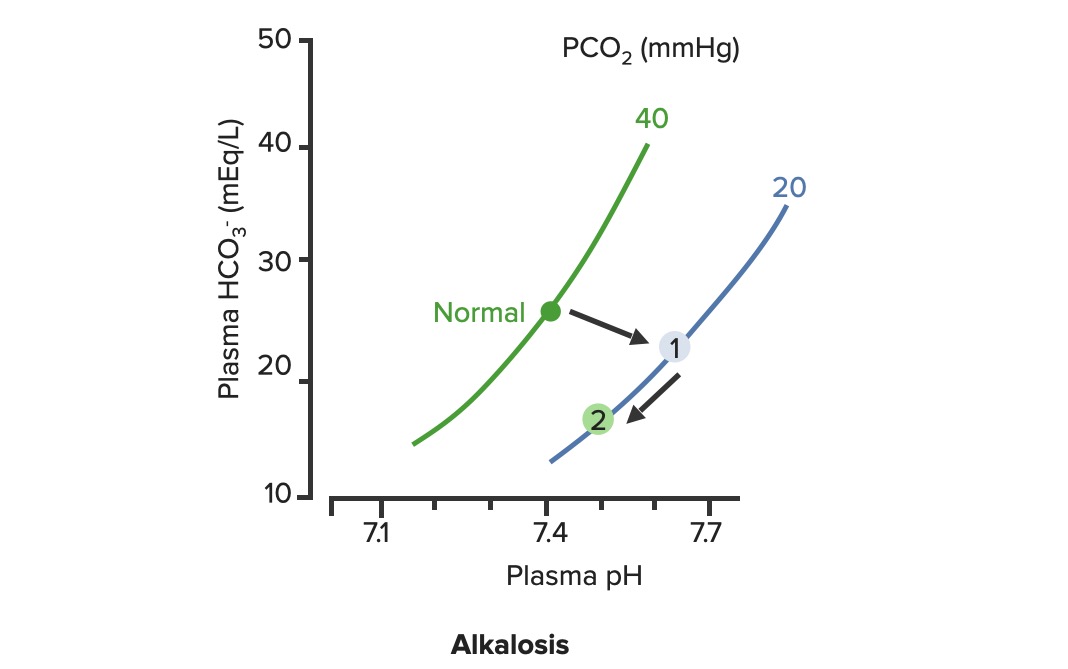
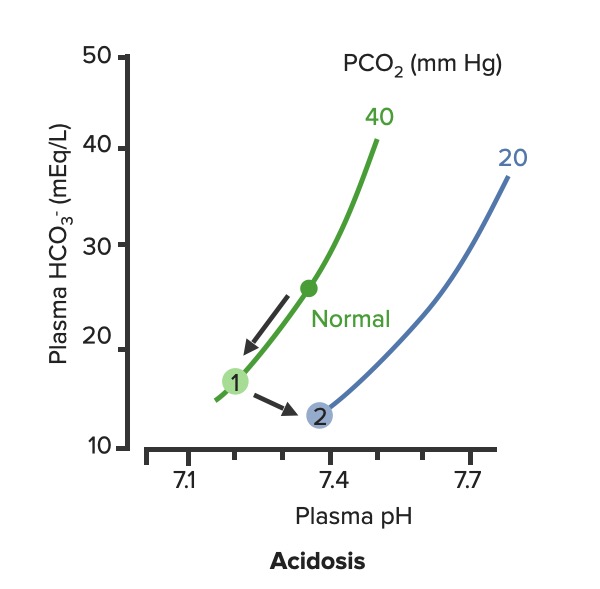
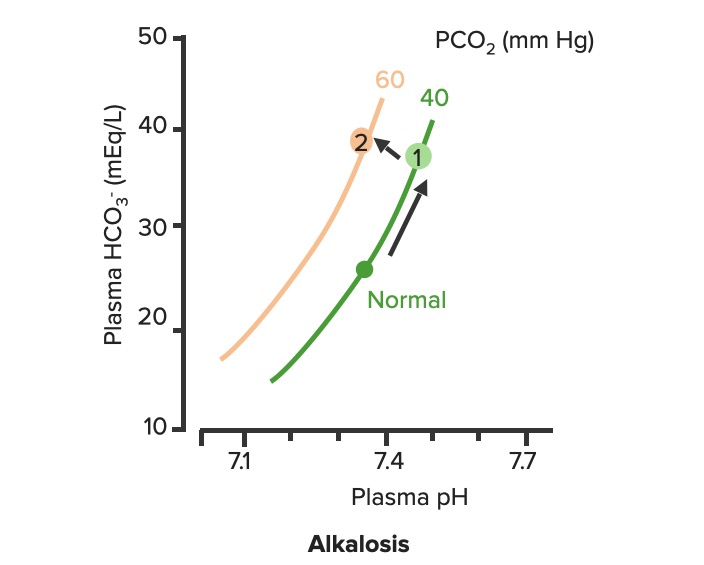
00:02 Let’s take a look at the topic of compensations. 00:05 A compensations seems as though that it should be an easy one because both are moving in the same direction but you never know as to whether or not there is proper degree of compensation and if there’s an underlying issue, so the next topic is going to be intense like they have been in the past but there’s going to be issues here that I know the medical students either don’t like or are quite unfamiliar and so therefore you might get turned off, don’t do that to me, right now, you really cannot. 00:36 I want you to walk through this and listen to what I have to say. 00:40 I’ll take you step by step as to why you want to do with the math that’s upcoming and when you want to clinically apply it and as to how frequently you may want to use it. 00:51 So the more number of practice attempts that you have as we shall do, the better off you’ll be. 00:56 So, let’s begin by first talking about what primary metabolic process – remember, the first two steps we're dealing with first, what is my arterial pH? Your second step was to figure out what my primary disorder was, was it metabolic or was it respiratory? Now, let’s say that you did find it to be a primary metabolic process. 01:21 Either it being metabolic alkalosis or it being metabolic acidosis, then what's your method of compensation – obviously, you only dealt with or left with respiratory. 01:32 Tell me about this respiratory compensations? A respiratory compensation or a primary metabolic arrangement is immediate. 01:40 It will improve the pH back towards the normal but it never receives or never gets to full compensation. 01:48 For example, if there is a metabolic alkalosis that’s taking place in your patient, which too much bicarb you are immediately going to start slowing down your breathing so that you can then hold on to you carbon dioxide, that is immediate compensation. 02:04 Let’s continue. 02:07 Now, this is one method by which you may be shown clinically and on your boards, as to how to deal with compensation and primary? Guess what – don’t lose focus, don’t panic and even though you had three different graphs here, you stick to the anchor. 02:26 What was that anchor that we talked about? The pH, okay. 02:31 So let’s take a look at the pH in our patient, shall we? What is it? At first all around the left side, in the middle graph, is your pH, and you find it to be well 7.4 but then what ended up happening? A drop, a severe drop in pH to 7.0. What happened? Oh, maybe there was consumption of alcohol like crazy, that’s a metabolic acidosis or maybe later on in salicylate poisoning. 03:00 I said later, with salicylates, because earlier its respiratory alkalosis but at some point maybe later on, because you’re ingesting acid, that could have taken place or maybe perhaps there was a diabetic ketoacidosis – interesting. 03:14 Those three examples that I just gave you there: alcohol, salicylates, and DKA are all what type of acidosis? Metabolic – good. 03:23 Now, all the pH had dropped to 7.0. What you’re looking for as far as it being primary. 03:30 Well, you'll find that your bicarb, take a look at bicarb, that is the top graph and you find it to be normal range at first, that’s the green bar that you see and you find that the bicarb decreased along with the pH so therefore, so far, what’s your disorder? It’s a primary metabolic acidosis – are we clear? Next, what do you expect to happen? This goes back to the general rule of thumb, what was that? Whenever there’s a decrease of one parameter, the other parameter also decreases, thus when there's a decrease in bicarb, you tell me, what you expect your carbon dioxide to do? Now, a lot of these, ladies and gentlemen, is just repetition, organization of thoughts, and just making sure that you have everything together, you’ll see why. 04:14 Okay, so they find your carbon dioxide to be decreasing as you see here in the bottom graph, right? In the bottom graph, you start finding that the carbon dioxide is decreasing. 04:26 How long did it take for this carbon dioxide to drop so quickly? Good, immediate. 04:31 How are you dropping your carbon dioxide, what’s your breathing pattern? Good, you’re increasing your breathing pattern – welcome to hyperventilation. 04:41 Okay, now are we done? No. The degree of compensation comes into play. 04:46 Take a look at that formula that you see there – PCO2 1.5 times bicarb plus or minus 8. 04:53 Okay. Now that’s something that you may want to memorize and often times medical student don’t or they don’t know how to use it. 05:01 My point is this, once you figure out that formula and at this point you find that the compensation, whose compensating in metabolic acidosis? The lungs are, by doing what? Blowing off carbon dioxide. 05:16 What's the degree of compensation that you should be finding with carbon dioxide? Limit of 10, meaning to say that the degree of compensation could be well a drop of approximately 30 of your carbon dioxide to compensate for the drop of the primary bicarb. 05:34 Listen to what I just said. At first it was the bicarb that changed. 05:40 Take a look at the formula – 1.5 times changed in bicarb. What’s the normal bicarb? Approximately 24, from this the change in your patient was what? A drop from 25 all the way down to 10 so approximately a change of 15 and when you do the math here, plus or minus 8, you will then come up – now trust me, you will get a formula that is quite simple to deal with but you have to understand the concept first. 06:11 Now that value that you’re going to turn out, listen, that value that you're going to turn out from that formula, what is it that’s compensating? In metabolic acidosis the carbon dioxide, once you churn out the value from the formula, remember, now you have to apply but what do you want to do with the carbon dioxide? All you’re getting is a value like an absolute value from that formula. 06:36 Do you want to take that value and add it to 40? That makes no sense. 06:42 You have metabolic acidosis, what's your bicarb? Decreased. 06:47 What do you want to do, how do you handle your carbon dioxide? You want to decrease it, don’t you? So therefore, that value that you get let’s say that you did get 30 as your value, you subtract that from the 40 and you get your 10, is what you’re looking at here. 07:04 So limit of ten, you drop by 30, so you take your 40 minus the 30, end up getting 10 as being the limit of your compensation. 07:13 Let’s move to another example. So this is where your degree of compensation becomes a little tricky so it’s important that you understand the concept first. 07:22 So let’s do the concepts first and I could assure you that many times, when you deal with such questions, that hopefully, you’ve come up with the answer along the way and if you have, fantastic; if you haven’t then you keep going to the next step, next step, next step, next step. 07:38 Guess what? We're not quite done with metabolic acidosis but this is just to begin conversations and then let’s do the same thing with metabolic alkalosis. 07:47 Step one, what is it? Oh, pH. 07:50 Well, take a look at the middle, our middle graph that you find there on your right is an increase in pH. 07:57 We can all agree it's alkalosis. 08:00 Next, well, now you try to figure out the primary, let’s go and take a look at bicarb. 08:05 You find an increase in bicarb above 25, it moved up to 40, what’s your primary disorder? Metabolic alkalosis. Now you tell me quickly, you see that things are becoming easier. 08:15 Compensation – what should the carbon dioxide do? Oh, same direction – carbon dioxide should be increasing so therefore what’s the patient doing? Hypoventilating, retain the carbon dioxide, are we clear about what the patient’s doing? Okay, now you want to try to figure out the degree of compensation. 08:34 What is it that you're gonna use here? Well the PCO2 at 0.9 then it’s the change in bicarb and plus or minus your 9. 08:45 When you end up getting such a thing, you’ll notice that here, that the imit is 60. 08:51 That means that when you got the value through the formula which gave you approximately 20, stop there. 08:56 Close your eyes, say that you figured out the value in the formula and you get 0.9 times your bicarb plus or minus 9 and you get 20, stop there. 09:06 Now, if you know that this is metabolic alkalosis, what do you want to do with that 20? Do you wanna add it to 40 or do you wanna subtract it from 40? You want both to increase, please understand, that you’re also going to build up carbon dioxide, therefore the limit is at 60, so whatever, once again, in both of these instances, whatever value that you come up with, you take that value either add it to carbon dioxide like you did here with metabolic alkalosis or you subtract it from carbon dioxide as you deal with metabolic acidosis. 09:39 But if your foundation isn’t strong and you don’t know as to what direction these gasses are moving, well, best of luck to you, but listen to what I’m saying. Let’s move on now. 09:49 Now, this is just the beginning conversation, degree of compensation –
About the Lecture
The lecture Approach to Acid Base Status: Step 3 – Laboratory Diagnostics by Carlo Raj, MD is from the course Pulmonary Diagnostics.
Included Quiz Questions
Which of the following occurs as a compensatory mechanism during a primary metabolic process?
- Immediate respiratory compensation.
- Delayed respiratory compensation.
- Immediate metabolic compensation.
- Delayed metabolic compensation.
What is the maximum amount of respiratory compensation that can occur during metabolic acidosis?
- 10 mmHg
- 100 mmHg
- 60 mmHg
- 16 mmHg
- 6 mmHg
Which of the following processes is compensated when the partial pressure of carbon dioxide is decreased? Select all that apply.
- Metabolic acidosis
- Respiratory acidosis
- Metabolic alkalosis
- Respiratory alkalosis
In metabolic acidosis, the degree of respiratory compensation is calculated by using which of the following formulas?
- PCO2 = 1.5 x [HCO3] + 8 +/- 2
- PCO2 = 15 x [HCO3] + 8 +/- 2
- PCO2 = 0.9 x [HCO3] + 9 +/- 2
- PCO2 = 1.5 x [HCO3] - 8 +/- 2
- PCO2 = 1.5 x [HCO3] + 18 +/- 2
What is the maximum amount of respiratory compensation that can occur during metabolic alkalosis?
- 60 mmHg
- 16 mmHg
- 40 mmHg
- 66 mmHg
- 10 mmHg
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
5 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |