Playlist
Show Playlist
Hide Playlist
COVID-19: Pathogenesis
-
Slides Coronavirus Pathogenesis.pdf
-
Download Lecture Overview
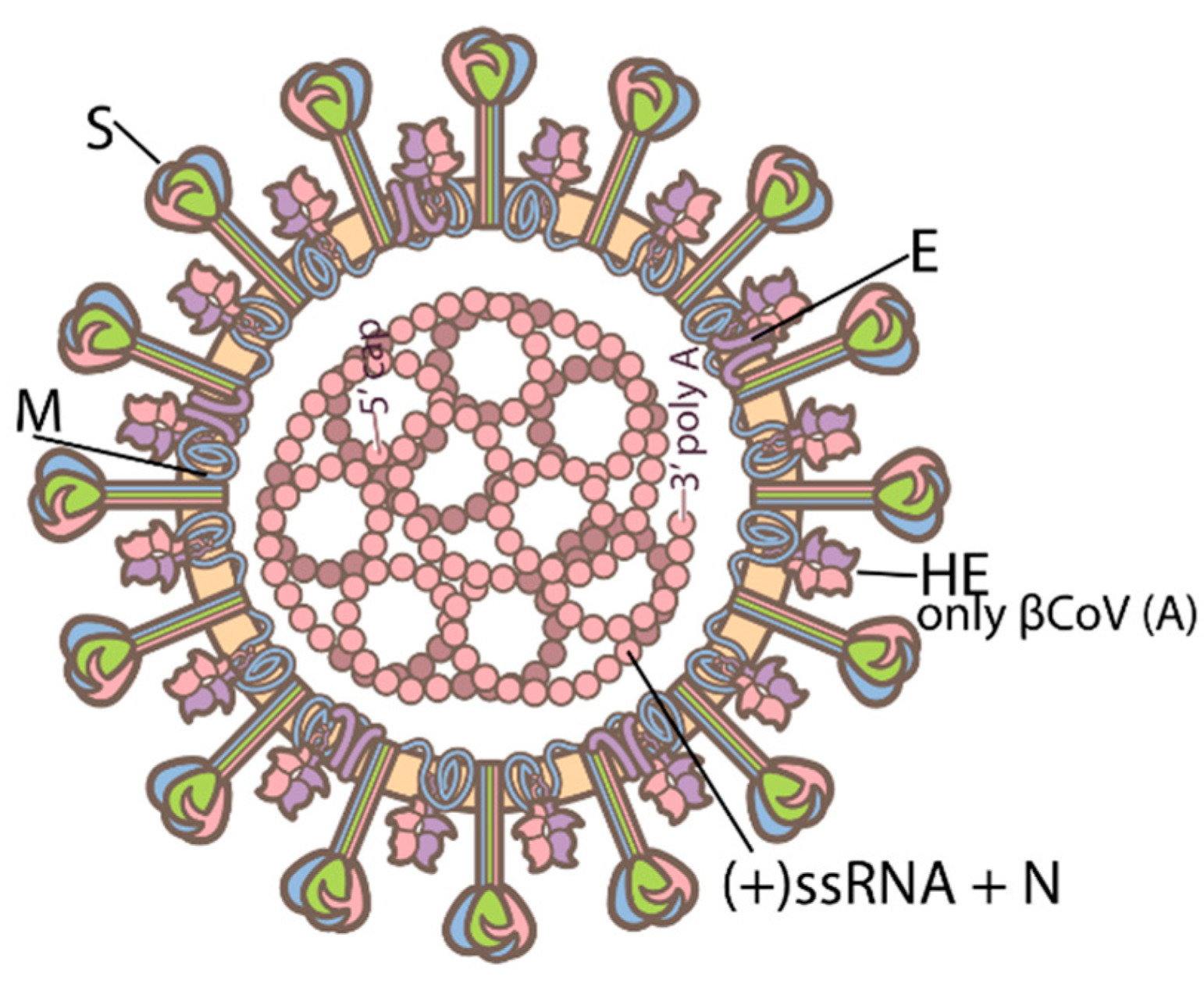
00:08 COVID-19 Pathogenesis. 00:11 There are two main processes that drive the pathogenesis of infection by SARS-Coronavirus II or COVID-19. 00:18 The first, is the early infection, this is driven by actual attack of the virus on its target cells and replication within those cells. 00:27 However, a secondary process a later infection or perhaps more accurately named, “Later inflammation,” is an exaggerated immune or inflammatory response, to that viral infection that further results in tissue damage or innocent bystander damage by the human immune system. 00:44 Typically, after exposure to the virus as you see here, the SARS-Coronavirus II, it will bind to its target cell and then its RNA, will become internalized. 00:56 There are two methods to enter the cell, either through membrane fusion and insertion of the virion or by endocytosis, if the cell has the ability to perform this act. 01:08 After endocytosis, the RNA is internalized and undergoes translation and transcription process, to both create more RNA for new virions, as well as then a synthesis of viral proteins, the four proteins discussed in another lecture, so that then the virus can be assembled within the cytoplasm of its target cell and then be released through a mechanism, which, remains to be determined. 01:35 The membrane fusion, typically occurs, through binding of the spike protein or the S-Protein of the SARS-Coronavirus II, to the ACE-2 receptor. 01:47 The SARS-Coronavirus II’s spike protein specifically recognizes and binds tightly to angiotensin-converting enzyme 2, now known as, the ACE-2 receptor for the virus. 02:02 After that initial binding then proteolytic cleavage of the S or spike protein must occur via host cell proteases, especially TMPRss2 and that is essential to allow that viral membrane, to fuse with its target cells membrane. 02:21 ACE-2, angiotensin-converting enzyme 2, is expressed robustly throughout the human body, especially by epithelial cells in a blood vessel, so, so-called endothelial cells, but also expressed by type II pneumocytes, in the lungs, elsewhere in the kidney, the intestine et cetera. 02:42 So, after the coronavirus enters into its host cell, via binding to the ACE-2 receptor or enzyme, the receptor the ACE-2 receptor is internalized and then is followed by a secondary inhibition of further ACE-2, expression by the target host cell membrane. 03:03 This release causes then ADAM17 gene expression, which, subsequently releases tumor necrosis factor, alpha and cytokines, so pro-inflammatory cytokines, especially such as the interleukins like 1 and 6 and then interferon gamma. 03:20 This, sets up then a vicious cycle of further ACE-2 inhibition, further gene expression, further release of pro-inflammatory cytokines and so eventually one can drive a cytokine storm. 03:33 High levels of ACE-2 expression, so, again receptors, for the virus itself are associated with pre-existing comorbidities, especially chronic cardiovascular disease and these individuals have a much higher risk of severe COVID-19. 03:48 There are other comorbidities as well, that have been identified clinically for example, individuals with insulin dependent diabetes, mellitus, individuals with hypertension and it may be, that these individuals also express higher levels of ACE-2, putting them at further risk of more viral binding. 04:07 What happens when ACE-2 is down regulated or suppressed? Well ACE-2 itself is a negative regulator, of the renin-angiotensin-aldosterone system or the are the (RAAS) system and if it is down regulated by viral binding that negatively or directly affects cardiovascular function. 04:29 Also, ACE-2 has a protective role in epithelial cells in the lungs, especially in the alveola and reducing its expression, will lead to alveolar cell damage. 04:40 Of course, we know, COVID-19, is primarily a respiratory illness and you can start to understand, why, when the target of opportunity of course is ACE-2 down regulation and allowing alveolar cell damage. 04:56 So, let's go through a typical immune response and this is not unique to SARS-Coronavirus II infection, this is how the human body's adaptive immune system responds to any antigenic challenge, any exposure to not self. 05:12 The adaptive immune response, starts with recognition and binding or opsonization if you will, of the antigen, in this case, SARS-Coronavirus II, by antigen presenting cells, “APC’s,” and these are macrophages dendritic cells and some epithelial cells, all of which are accompanied by cytokine release. 05:34 After ingestion of that antigen, the antigen presenting cell, then expresses the antigen at its cell surface in combination with MHC classes I and II to a T-lymphocyte a T-cell such as you see here, binding to the T-cell receptor the TCR. 05:53 Although there are many subsets of T-lymphocytes, the two that we're focusing on here, are the biggest categories the CD4+ T-lymphocytes or T-helper lymphocytes and the CD8+ or cytotoxic T lymphocytes. 06:09 These, are then activated followed by binding or perhaps concurrently by binding of the antigen presenting cell, with the B-lymphocyte. 06:18 The B-lymphocytes interactions with the CD4 T-helper, T-lymphocytes, then allows formation of antibodies, all uniquely specific to the initial antigen, expressed by the antigen presenting cell, again, in this case, SARS-Coronavirus II and most often the spike protein, since that is the most easily accessible antigen, which is recognized by the antigen presenting cells. 06:43 B-lymphocytes, will produce antibodies and a small subset of those specific B-lymphocytes, will then, mortalize, into what are called “Memory cells,” and this is one of the joys or the benefits of the adaptive immune response. 06:58 It remembers its response so it can reactivate and escalate should there be a new challenge to that same antigen. 07:07 So, how does all this look then and the more macroscopic level? Again, as we, as humans, inhale and or are exposed to SARS-Coronavirus II, we enter the virus into our lungs, were it finds its way to ACE-2, expressing alveoli, in epithelial cells in the alveolus and there, that cycle, we just discussed, can precipitate a cytokine storm. 07:34 This, allows for leakage of fluid, of red blood cells, of further phagocytes such as neutrophils and macrophages, into the alveolar space and eventually, as this immune cytokine storm-based response develops, the alveolus floods and it is unable to allow for normal ventilation and perfusion and thus gas exchange does not occur. 07:59 This as we know leads to the shortness of breath and the respiratory failure, which, so typifies severe COVID-19.
About the Lecture
The lecture COVID-19: Pathogenesis by Sean Elliott, MD is from the course Coronavirus.
Included Quiz Questions
How does the SARS-CoV-2 virus enter the host cell via membrane fusion?
- Proteolytic cleavage of the viral S protein permits fusion of the virus to the host cell membrane, leading to internalization of the virus.
- Viral proteases cleave the ACE-2 receptor, leading to the internalization of the virus into the host cell.
- Phages attach to receptors on the host cell and insert the viral genome.
- The viral genome is injected into the host cell after binding to the surface M protein.
- The viral envelope protein binds to the ACE-2 receptor and proteolytic cleavage leads to internalization of the virus.
What type of cells most abundantly express ACE-2 receptors in the body?
- Type II pneumocytes
- Skin epithelial cells
- Nasal mucosal epithelium
- Vascular endothelium
- GI tract cells
After the SARS-CoV-2 virus binds to the cell and is internalized, what is the host immune response to infection?
- Cytokine release of interleukins, interferons, and tumor necrosis factors
- Release of angiotensin-converting enzyme
- Activation of renin-angiotensin system
- Production of antibodies by T cells
- Production of memory cells to the antigen-presenting cell by T helper cells
After infection with SARS-CoV-2, what happens in response to the cytokine storm?
- Leakage of fluid into the alveolus disrupts gas exchange, causing respiratory symptoms.
- Blood pressure drops in response to ACE inhibition.
- Blood pressure drops in response to activation of the renin-angiotensin-aldosterone system.
- Cytokines cause improved gas exchange in the alveolus in response to respiratory distress.
- Cytokines stimulate the recruitment of immune cells to the respiratory system to help fight the infection.
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
1 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |
1 customer review without text
1 user review without text