Playlist
Show Playlist
Hide Playlist
Acids, Bases and Buffers
-
Slides 07 pHBuffers AcidBaseBalance GeneralPhysiology.pdf
-
Download Lecture Overview
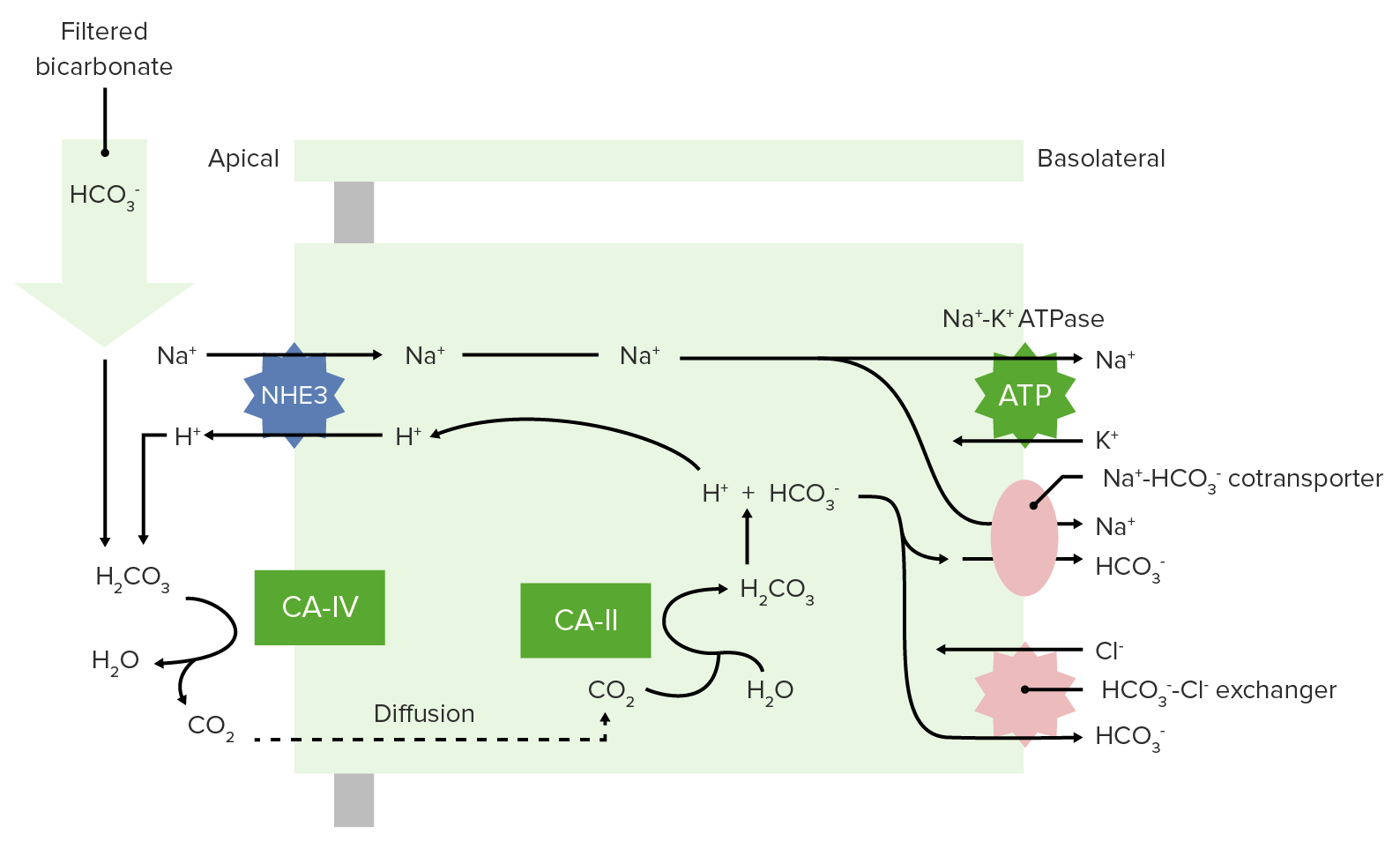
00:01 The types of acids that you could get. So, these are where, what type of acid it might be. 00:08 First you have Volatile acids. 00:11 Volatile acids are primarily carbon dioxide. 00:14 So, you don’t always think of carbon dioxide as an acid but it really is. 00:19 It fits in to the carbonic and hydrase equation and if you have CO2 plus water, forms carbonic acid and then, will this associate into hydrogen ion and a bicarb. That process is acidic. 00:38 So, carbon dioxide in this case is acting like an acid. 00:43 Non- volatile acids, these are stabler but these are harder for the body to deal with. 00:49 A volatile acid is easy for the body to deal with. You just blow off CO2. 00:55 But a Non- volatile acid has to be not only taking care of but usually at the level of the kidney. 01:02 We have things like sulfuric acid, phosphoric acid and you even have some organic acids associated with different types of amino acids. 01:13 You have a very small amounts of bases that are added to the system. 01:17 This is mainly from your gastrointestinal tract. 01:21 You do have buffers. Buffers are going to be very important. 01:27 You can hold on to a hydrogen ion or release it as a buffer. 01:32 So it really acts to stabilize the pH in a narrower range. 01:38 Our big buffers that we use in the body; the first one is bicarbonate. 01:43 And bicarbonates going to be circulating around the blood, it’s going to bind majority of the hydrogen ions. 01:50 but we also do have some non- bicarb base buffers. 01:53 Things like phosphates, things like proteins these can also buffer hydrogen ions. 02:00 Probably, not as important as bicarb but it’s part of the overall buffering process. 02:07 And so, we need to discuss them. 02:09 Some cells we utilize by certain buffering techniques or types more than others. 02:18 So, let’s just go through a couple example pH changes. 02:22 This way we can get a better insight into how the pH will change when you add an acid or add a base. 02:31 So, let’s first add an acid. 02:33 So let’s say we add a certain amount of an acid to a beaker. 02:37 Then we wait until that particular beaker equilibrates. 02:43 In this case, you’ll see that there are more hydrogen ions around which will decrease the pH. 02:50 In our example here of the acid that was added, we lowered pH by 0,03. 02:57 It doesn’t seem like a big pH change, right? 0,03 it’s not even 1 right? But think arterial blood needs to have a pH at 7,4 plus or minus (±) 0,05. 03:13 So a 0,3 change could take it out of homeostatic normal. 03:20 If we add now a base, rather than an acid, we put it in to our beaker, we sit in let it equilibrate and we have an end solution. 03:31 We take the pH of that and we maybe give a ph change of an increase of 3. 03:38 Again, it doesn’t seem like a much to have a 0,3 increase. 03:42 But again, that can take you out of your homeostatic normal. 03:46 And therefore, you would be in an alkalimic condition. 03:52 So, whether you add an acid or add a base, you have to think about how that is going to change your pH. 04:01 What acids could we add? Well, you could add a volatile one like CO2. 04:07 You can add a non- volatile one like something like a sulfuric acid or from uric acid. 04:13 But if you want to do something like add a base, therefore it’s probably coming from the GI tract but it’s gonna be something with an OH kind of group attached to it.
About the Lecture
The lecture Acids, Bases and Buffers by Thad Wilson, PhD is from the course Acid-Base Balance.
Included Quiz Questions
What are non-volatile acids?
- Complete or incomplete byproducts of metabolism
- Buffered with bicarbonate
- Also called non-fixed acids
- Eliminated by the lungs
- Mostly derived from the intestine
What happens if the amount of CO2 in arterial plasma doubles, the log of 2 is 0.3?
- pH will fall to 7.1, or 0.3 points.
- pH will rise to 7.7, or a 0.3 point rise.
- Respiratory alkalosis
- Metabolic acidosis
- A significant change in [HCO3-]
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
5 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |