Playlist
Show Playlist
Hide Playlist
Vitamin B12
-
Slides VitaminsK,E,B12,FolateReady Biochemistry.pdf
-
Download Lecture Overview
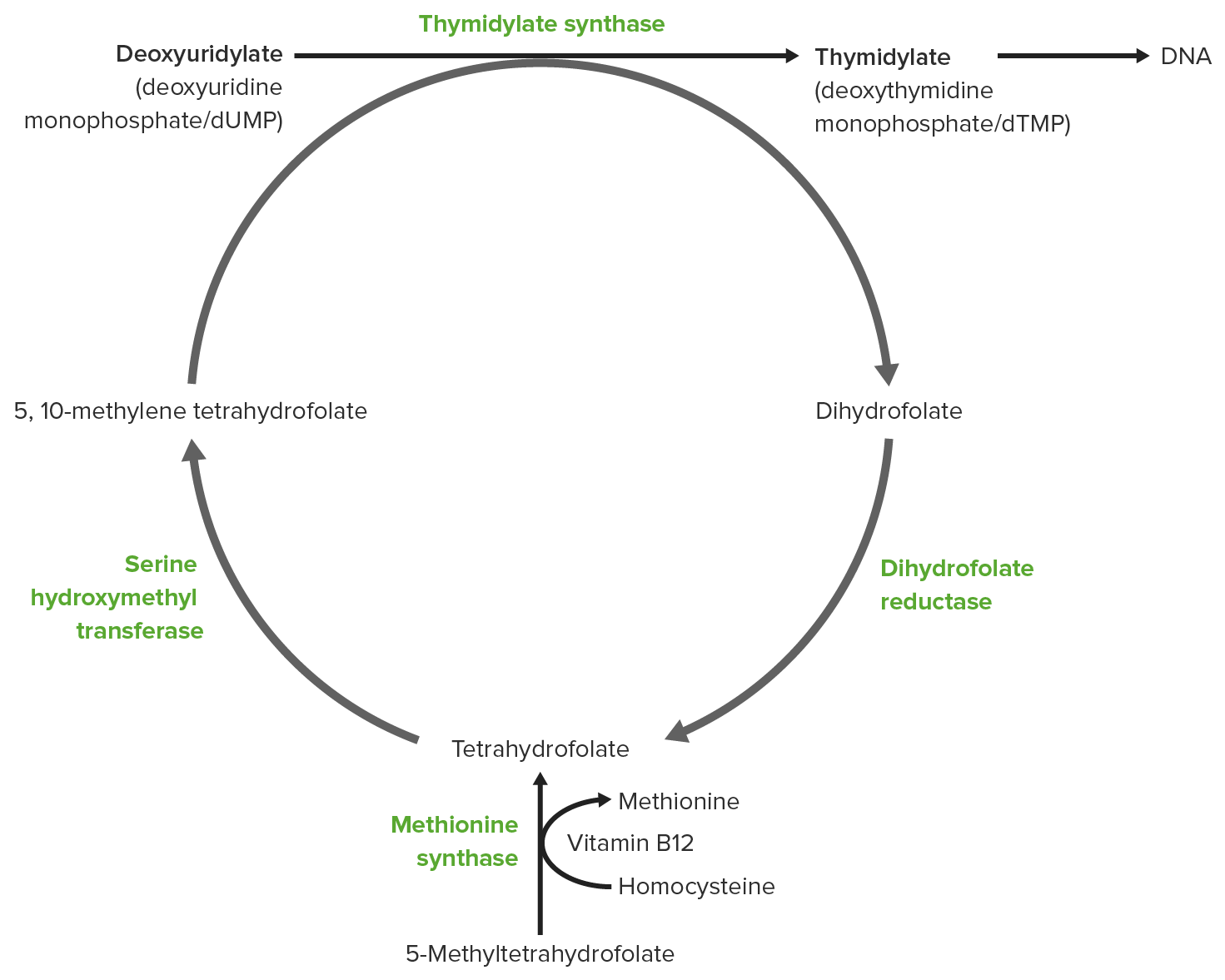
00:01 Vitamin B12 is by contrast of vitamin that’s very unusual in its structure. 00:07 You can see one of the forms of vitamin B12 on the screen here. 00:10 And it participates in a very unusual set of reactions. 00:14 Now, it turns out that these unusual reactions that it participates in and that it helps to contribute to are very critical. 00:21 Absence of vitamin B12 results in various health issues. 00:26 Cyanocobalamin is one of the forms of vitamin B12 that is made and you can see them on the screen here. 00:32 An artificial form is made by human beings, but given the people who lack vitamin B12. 00:39 And It functions perfectly as vitamin B12 and there are various forms of vitamin B12 that are naturally produced as well. 00:46 Now, one of the critical things about vitamin B12 is that it’s not made in plants. 00:51 So people who are one on strict vegan diets, who are not eating any animal products will become deficient overtime with vitamin B12 because they can’t get what they need from plants. 01:01 People who are on diets like that need to be sure they check with their doctor and get supplements as appropriate to get enough vitamin B12 to maintain the reactions we’ll describe here. 01:10 Vitamin B12 is normally obtained in a diet from meat and dairy products or other animal products and it doesn’t take a lot. 01:18 Deficiency of vitamin B12 leads to pernicious anemia which is a very severe anemia relating to your blood supply. 01:25 And people who sometimes lack energy, one of the things they'll check in a person is whether vitamin B12 level's okay. 01:32 Because people with vitamin B12 deficiency will first notice that they don’t have energy because of lack of that vitamin. 01:40 There are several different forms of vitamin B12 as I said. 01:44 Methylcobalamin is found in the cytosol of cells. 01:48 Adenosylcobalamin which is a form of cobalamin in which the adenosyl group of ATP is linked to cobalamin is found in the mitochondria. 01:56 And the bioactive forms of vitamin D are needed for the metabolism of a few compounds. 02:02 One is the synthesis of methionine. 02:04 The second is handling propionic acid which is present in our cells. 02:09 And third is for the formation of neurotransmitters. 02:13 Now, I want to show you here the reaction where vitamin B12 is necessary for the metabolism of an unusual compound called propionic acid. 02:23 Vitamin B12 is important because what it does is it allows the cell to be able to break difficult to break single carbon bonds and move that carbon from one position to another. 02:35 And the reaction I’m going to show you here is a pretty unusual one. 02:38 In the set of reactions that you can see here, propionyl CoA on the left is a three-carbon molecule. 02:44 It is being converted into a 4-carbon molecule on the right known as succinyl CoA. 02:49 Succinyl CoA is a metabolite in the citric acid cycle. 02:52 So the goal is getting from this 3-carbon propionyl CoA to the 4-carbon succinyl CoA and the cell goes about it in an unusual way. 03:00 The first step in the process is to add a carboxyl group from carbon dioxide using propionyl CoA carboxylase. 03:08 That is the enzyme that catalyzes the first reaction and it puts that carboxyl group that you can see in the square on the central carbon of that three-carbon propionyl CoA making methylmalonyl CoA. 03:21 Well, methylmalonyl CoA cannot be metabolized as such. 03:25 That molecule has to be rearranged before it can become succinyl CoA and that rearrangement uses an enzyme known methylmalonyl CoA mutase that requires vitamin B12. 03:38 And that reaction itself is unusual because that carboxyl group is in the wrong stereolized numeric configuration to start. 03:46 It has to be moved essentially from the right side of the molecule to the left side of the molecule and then that carboxyl group has to be moved to the top to get to where it is in the succinyl CoA. 03:57 Vitamin B12 is critical for that feature because vitamin B12, as I said earlier, can break those carbon-carbon double bonds and move things around. 04:06 Vitamin B12 is also important in the sense that has an atom of cobalt in. 04:12 It is the only compound in our body that contains cobalt. 04:15 And that cobalt is important for that function to hold onto the carboxyl group in this moving around process. 04:23 So vitamin B12 is important for the synthesis of methionine from aspartic acid. 04:29 If you remember from the amino acid metabolism lecture, methionine is in the aspartic acid family of compounds. 04:36 So we can start with the aspartic acid and get all through to methionine and the very last reaction of that process requires vitamin B12. 04:44 Let’s take a look at the reactions occurring to go from aspartate to methionine. 04:49 The first step of aspartate is phosphorylated producing the aspartyl beta phosphate as you can see here. 04:55 Reduction of that produces beta aspartate semialdehyde as you can see, followed by another reduction to produce the intermediate shown in the central part of this figure. 05:06 At that point, an unusual addition is made and that succinyl CoA is joined to this molecule to make an even bigger molecule. 05:14 In the next reaction, cysteine replaces the succinate that had been added into the last molecule. 05:20 Now this turns out to be an important reaction because cysteine is a source of sulfur for making methionine. 05:26 Cysteine and methionine are the only two amino acids that contain sulfur. 05:29 So the sulfur source is essential for making methionine. 05:33 That creates the compound known as cystathionine as you can see here. 05:37 We’re getting very close to having methionine. 05:40 Breakdown of cystathionine yields three products. 05:43 Pyruvate as you can on the top and ammonium ion which is involved in the loss of the amino acid in the process and finally homocysteine as you can see on the right. 05:55 This reaction, I’m showing some detailing because it’s the reaction that requires vitamin B12 in the synthesis of methionine. 06:02 It also requires interaction of two folates. 06:05 The folates are the source of the extra carbon that is added during the synthesis process. 06:11 So the carbon source for the extra carbon, this methyl group that's being added, is N5 methyltetrahydrofolate. 06:19 Folates are involved in one carbon metabolism just like vitamin B12 is. 06:23 But vitamin B12 is involved in handling those carbons and the folates are sources of the carbons. 06:30 So the source here is as noted. 06:32 The enzyme performing this catalysis requires B12 as coenzyme. 06:37 Without B12, this reaction cannot occur and we can’t make methionine.
About the Lecture
The lecture Vitamin B12 by Kevin Ahern, PhD is from the course Vitamins. It contains the following chapters:
- Vitamin B12
- Bitamin B12 - Propionic Acid Metabolism
- Vitamin B12 and Methionine Metabolism
Included Quiz Questions
Which of the following statement is not true regarding vitamin B12?
- A vegetarian diet is a rich source of vitamin B12, followed by dairy products.
- Vitamin B12, cobalamin, is a water-soluble vitamin essential for the normal functioning of the nervous system.
- The prokaryotic organisms are capable of synthesizing vitamin B12, whereas single-celled and multicellular eukaryotic organisms are unable to synthesize it.
- Vitamin B12 contains a biochemically rare element cobalt in the center of the planar tetra-pyrrole ring.
- Vitamin B12 plays an essential role in the formation of RBCs, the synthesis of DNA, and the metabolism of fatty acids and amino acids in every cell of the animal body.
Pernicious anemia is caused by which of the following?
- A deficiency of vitamin B12
- High doses of vitamin B12
- Absorption of an abnormal biochemical type of B12
- High doses of hydroxocobalamin
Which of the following is not matched correctly?
- Pernicious anemia — deficiency of vitamin C
- Methylcobalamin — present in the cytosol of cells
- N5-methyl tetrahydrofolate — source of extra carbon during methionine synthesis
- Vitamin B12 — handles propionic acid metabolism in the cell
- Adenosylcobalamin — found in the mitochondria of the cells
Which of the following is a vitamin B12-dependent enzyme?
- Methylmalonyl-CoA mutase
- Catalase
- Glutamate carboxylase
- Vitamin K epoxide reductase
- Methionine reductase
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
5 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |