Playlist
Show Playlist
Hide Playlist
Folate Metabolism and Recycling
-
Slides VitaminsK,E,B12,FolateReady Biochemistry.pdf
-
Download Lecture Overview
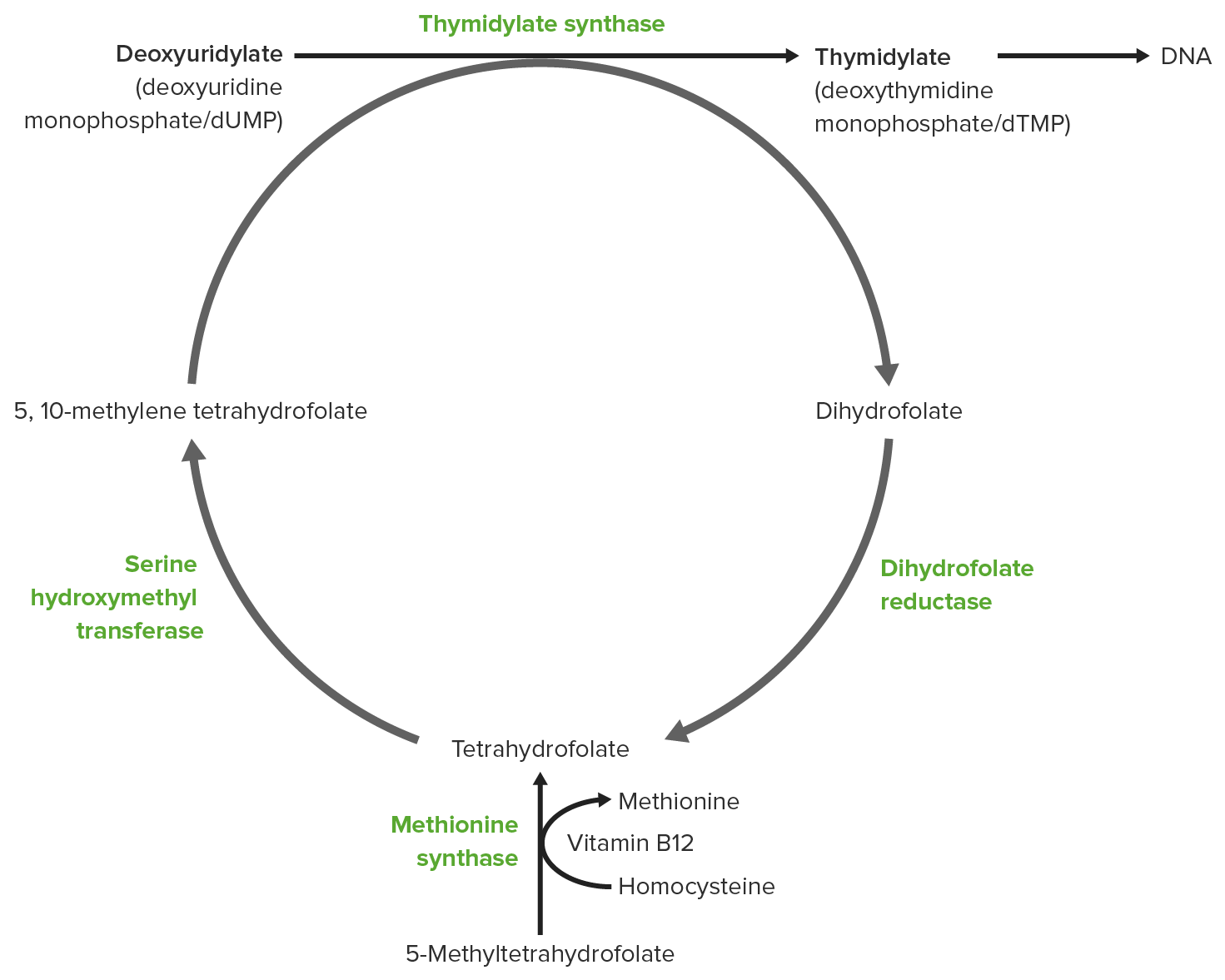
00:01 Folates are important compounds that donate one carbon units to individual biochemicals that are being synthesized. 00:09 And we will see how that occurs here. 00:11 So folates are involved in single carbon transfer reactions. 00:15 They are sometimes called vitamin B9, although more commonly refer to them as folic acid or folates. 00:21 Deficiencies of folates in our diet can be very severe, resulting in megaloblastic anemia in adults, cardiovascular disease, birth defect in infants, particularly failure to close the neural tube during the process of development. 00:37 And these problems overcome to a large extent in recent years by supplementation of folates in grains. 00:44 It's things such as our bread now contains folate that didn’t contain folates before. 00:48 You can see two of different folates on the right and as we'll see, this is a whole family of compounds but slightly different structures involved in adding different forms of one carbon units to build a biochemical molecule. 01:00 There are many different forms of folate as I noted and they can differ in both their oxidation state and also in the configuration of carbons they have to allow diversity of forms of one carbon units that can be added to a growing biochemical molecule. 01:16 Now, folates are important in many metabolic processes. 01:19 We see them, for example, as required in the synthesis of purine nucleotides for making RNA and DNA. 01:25 They’re also necessary for making thymidine from the uracil containing nucleotides. 01:30 We've seen how they’re involved in other lectures here in the synthesis methionine. 01:35 And they’re also important for the interconversion of serine and glycine in the metabolism of those two amino acids. 01:43 You can see two different forms of folates: the N5, N10 methylenetetrahydrofolate on top and the 5-methyltetrahydrofolate on the bottom. 01:53 Now, if we look at the serine and glycine metabolism, we see one of the ways in which the folates are actually interchanged. 02:00 You can see the reaction as described on top where serine is interacting with tetrahydrofolate to create glycine and N5, N10 methylenetetrahydrofolate. 02:10 Well, here’s what it actually looks like and what’s going on. 02:13 Now remember that this had a fairly long chain and I don’t have enough room on the slide to show you the long chain. 02:18 So I’ve abbreviated it by putting an R on the right side of the THF molecule. 02:24 The reaction that is important here occurs within that molecule that you can see on the screen. 02:29 But in this reaction, serine is actually donating a carbon to the tetrahydrofolate. 02:35 And we can see that carbon has been added between the nitrogens, that are on the right side of that molecule In this case, the addition is a methylene group, the CH2 and the product of that reaction is the amino acid glycine. 02:50 Meaning that after serine has donated its group, glycine is what results. 02:55 Well, this turns out to be a reversible reaction If the cell needs to make tetrahydrofolate, it can convert glycine into serine and then convert N5, N10 methylenetetrahydrofolate into folate. 03:07 So this can go either way depending upon the cell’s needs. 03:13 Folates are needed in purine metabolism, where purine nucleotides are being synthesized. 03:18 This reaction is a fairly mouthful of reaction that you can see on the screen, that I’m not going to read to you here. 03:23 In this reaction, another mouthful molecule as you can see combines with another folate to make an additional long named molecule. 03:33 That is not important here. 03:34 What’s important is actually what’s being added. 03:36 And what’s being added in each case is a formyl group. 03:39 A formyl group, you remember, is an aldehyde and we can see that aldehyde being added to the growing chain in the green box on the upper right. 03:47 We can also see the similar reaction occurring on the bottom and there is the addition of aldehyde that’s occurring. 03:53 We can see that in the bottom reaction, we’re getting very close to synthesizing a complete intact purine molecule. 04:01 Now, folates, as I said, are very important in the synthesis of thymidylate nucleotides from uracil-containing nucleotides and this is a very interesting reaction. 04:10 It looks a lot more complicated than it really is. 04:13 We see on the left, on the top, one of the folates, so the folate is involved in this reaction. 04:19 We see the uracil-containing nucleotide on bottom. 04:23 There, in the green square, is the methylene group that is actually added to the uridine-containing nucleotide as the reaction precedes to the right. 04:33 The intermediate is shown here and what happens in this process is we see this methylene group that becomes attached to the uracil and the attachment is shown on the molecule on the right. 04:46 There is that. 04:47 Now what’s happened here is the methylene group has been added, but it has been converted into a methyl group which requires an additional reduction. 04:56 The reduction comes from the oxidation of the tetrahydrofolate. 05:01 So we started from tetrahydrofolate after we oxidize it or ending up with dihydrofolate. 05:07 And that turns out to be very significant. 05:10 So the formation of dihydrofolate is a bit of a problem for the cell because the formation of dihydrofolate is a dead end. 05:19 Dihydrofolate cannot readily be converted back into the other folates. 05:23 We will see that process in just a little bit unless itself is changed. 05:28 This reaction has to recycle dihydrofolate. 05:31 We will see that there is an enzyme involved in this process. 05:35 Now, dihydrofolate looks like this and again, the structure is an important consideration here. 05:40 The important thing is dihydrofolate must be reduced. 05:43 Remember it got oxidized. 05:45 Dihydrofolate has to be reduced back to tetrahydrofolate. 05:49 And the enzyme that catalyzes this reaction is known as dihydrofolate reductase. 05:54 It uses the electrons, NADPH, to create tetrahydrofolate. 05:59 And as long as everything is preceding normal, this reaction goes along just fine and the cell replenishes is tetrahydrofolate after it makes thymidine nucleotides. 06:09 Well, this enzyme turns out to be the target for an anticancer drug. 06:13 And this target works because it messes with the nucleotide metabolism of cancer cells. 06:20 Tetrahydrofolate, of course, is something that can readily be converted into the other folates unlike dihydrofolate. 06:26 So once this tetrahydrofolate has been regenerated by this enzyme, everything is fine. 06:31 The target of this medication that is used to target this enzyme, dihydrofolate reductase, is known as methotrexate. 06:39 And as you look on methotrexate, you see that it looks very similar to the dihydrofolate that's on the left. 06:45 It turns out that methotrexate is a competitive inhibitor of the dihydrofolate reductase enzyme. 06:51 In the presence of methotrexate, the enzyme will not function. 06:55 And when the enzyme does not function, then tetrahydrofolate can’t be made. 06:59 Dihydrofolate is left as a dead end. 07:01 Well, after a few cycles of making thymidine nucleotides, this cell will run out of its folate so will convert everything into dihydrofolate and it can’t be produced outwards. 07:11 The reason that methotrexate works in some cases as an anticancer drug is because rapidly dividing cells are needing nucleotides faster. 07:20 And if you give cells that are rapidly dividing methotrexate, for a short period of time, they will be much more likely to die then cells that are dividing more slowly and don’t have such a rapid need for nucleotides. 07:32 Methotrexate is an important drug in this medication. 07:35 It’s a fairly severe drug because it’s using a hammer to really knock the cell but for certain cancers that are very aggressive, this may play an important role.
About the Lecture
The lecture Folate Metabolism and Recycling by Kevin Ahern, PhD is from the course Vitamins. It contains the following chapters:
- Folate Metabolism
- Folates in Purine Metabolism and in Thymidylate Synthesis
- Folate Recycling
Included Quiz Questions
Which statement is not true regarding folic acid?
- Folate deficiency leads to excessive hemorrhage in adults.
- Folic acid is given to pregnant women as a supplement to prevent neural tube defects.
- Folic acid is an essential vitamin and is required for the synthesis of nucleic acids and amino acid metabolism.
- Folate deficiencies can lead to severe megaloblastic anemia in adults.
- Vitamin B9 plays an essential role in the synthesis of thymidine from the uridine-containing nucleotides.
The folate derivatives act as substrates in which of the following reactions?
- Single-carbon transfer reaction
- Two-carbon transfer reactions
- Three-carbon transfer reactions
- Nitrogen-containing ring transfer reactions
- Cyclic hydrocarbon transfer reactions
Dihydrofolate reductase (DHFR) is often targeted in cancer treatment. Why?
- The inhibition of DHFR by folate antagonists (e.g., methotrexate) results in a deficiency in the cellular pools of thymidylate and purines and thus in a decrease in DNA synthesis.
- DHFR inhibition interrupts the formation of the mitotic spindle of cancer cells.
- DHFR inhibition causes lysis of the nuclear membrane in cancer cells.
- DHFR inhibition causes lysis of cancer cell membranes.
- DHFR inhibition interferes with cell membrane synthesis in cancer cells.
Which of the following reactions correctly describes the production of N5, N10-methylene-tetrahydrofolate?
- Serine + tetrahydrofolate (THF) ↔ glycine + N5, N10-methylene-THF
- Thymidylate + THF → serine + N5, N10-methylene-THF
- Glycerol + dihydrofolate → serine + N5, N10-methylene-THF
- Serine + dihydrofolate ↔ glycine + N5, N10-methylene-THF
- THF + glycine → serine + N5, N10-methylene-THF
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
2 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |
Dr. Ahern, I appreciate the pharmacological and pathophysiologic connections you make when delivering your lectures. It aids retention and usefulness. I am a nurse practitioner student and can also see the clinical connection. I have administered Methotrexate to many patients. Thank you.
Pure gold indeed !!! Presentation of chemical compounds during presentations is very helpful.