Playlist
Show Playlist
Hide Playlist
Body Water Balance: Terms and Definitions
-
Slides Water Balance Hypo and Hypernatremia.pdf
-
Download Lecture Overview
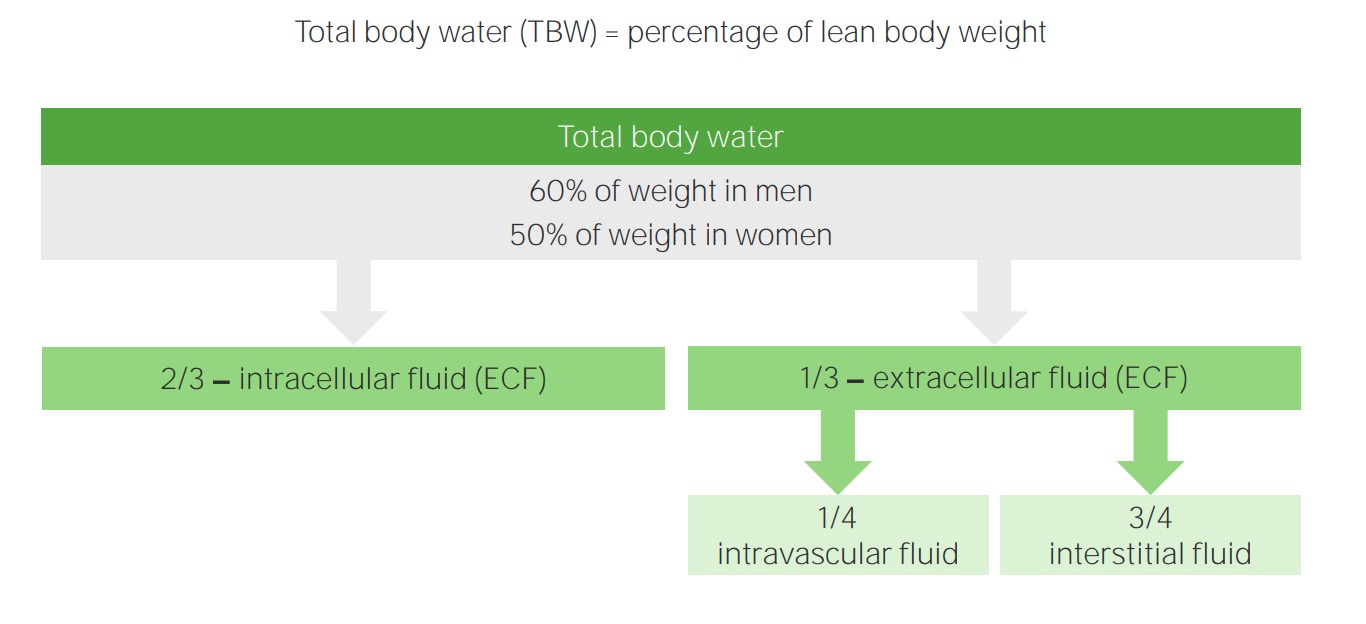
00:01 Hello, and welcome back to the nephrology curriculum. 00:04 Today, we're gonna be talking about water balance in the dysnatremias. 00:07 So, when we're talking about disorders of water balance, we're talking about hyponatremia and hypernatremia. 00:15 They're very common clinical problems and you should know about them. 00:18 And although the serum sodium level is abnormal, these clinical syndromes reflect an abnormality in water balance that may or may not be accompanied by changes in sodium balance. 00:30 Disorders of water balance are characterized by abnormalities in the concentration of serum sodium. 00:36 So, hyponatremia means that we have too much water, hypernatremia reflects too little water, and each of these particular disorders can exist at any level of total body sodium. 00:50 So, in order to understand this concept, it's really important to discuss how total body water is distributed. 00:57 When we think about total body water, that's the percentage of lean body weight. 01:02 So, in men, because they have more, traditionally, muscle mass or leaner muscle mass than women, 60% of their weight is total body water. 01:12 In women, about 50% of their weight is considered total body water. 01:16 The way that that distributes is about two-thirds of total body water will be contained in the intracellular fluid compartment. 01:24 Only one-third resides in the extracellular fluid compartment. 01:28 Of that one-third, three-fourths of that will stay within the interstitial fluid, and only about one-fourth is actually circulating in the intravascular fluid compartment. 01:38 Now, let's take an example. 01:40 If we have an 80-kilogram man, then if we say 60% of his weight is total body water, he has about 48 liters of total body water. 01:51 Now, remember, two-thirds of that is going to be in the intracellular compartment. 01:55 That means 32 liters are actually residing within the cells. 01:59 We said that one-third is going to be in the extracellular fluid compartment, or 16 liters will reside within the extracellular fluid compartment. 02:07 Of that, we have 12 liters within the interstitial fluid and only four liters is actually circulating within the vascular volume. 02:16 Now, the next concept that's really important when it comes to water balance is to really understand the difference between osmolality and tonicity. 02:25 So, plasma osmolality is really a measure of solute concentration. 02:30 It's determined by the ratio of plasma solutes and plasma water. 02:35 The majority of plasma solutes are things like sodium salts. 02:38 The remainder potassium, calcium, and glucose, and urea played less of a role. 02:43 Normal plasma osmolality is somewhere between 275 to 290 milliosmoles per kilo. 03:04 Now, glucose and urea contribute only a small amount to the plasma osmolality when they're within the normal range. 03:10 If they're abnormal, they can actually contribute quite a bit more. 03:14 Plasma tonicity is what we term the effective plasma osmolality. 03:20 That's the parameter that's sensed by osmoreceptors, and it determines the transcellular distribution of water. 03:27 Let's talk about that a little bit more. 03:30 So, plasma osmolality is gonna include osmotic contribution of urea, which is an ineffective osmol, since it can move freely across cell membranes. 03:41 So, it has very little effect on water movement when it's moving between these two cellular compartments. 03:47 Plasma tonicity on the other hand, which is the effective plasma osmolality, really reflects the concentration of solutes that do not easily cross the cell membrane. 03:57 So, things like sodium salts, for example, that will actually affect the distribution of water between cells and the extracellular fluid compartment. 04:06 So, this is an illustration that really diagrams what we're talking about here. 04:11 So, in the top compartment or the top part of the diagram, you can see the green which represents our intracellular fluid compartment, that's our cell, and then the ECF, which is adjacent to it on the left. 04:22 In our normal state, our plasma osmolality is somewhere between 275 and 290 milliosmoles per kilo. 04:29 When we're in steady state, water is gonna move freely between these compartments. 04:33 However, if we have a situation where we become hyperglycemic, then we have glucose that's added to the extracellular fluid compartment. 04:42 Now, remember, that's an effective osmol. 04:44 It cannot move across that cell membrane freely. 04:47 Therefore, the glucose exerts a tonic effect on water, and water will then move from the intracellular compartment to the extracellular compartment. 04:57 So, again, that's tonicity. That water is moving from that ICF to the ECF, because of the presence of an effective osmol in that extracellular fluid compartment. 05:08 And again, that's what's outlined in that left lower quadrant. 05:12 Now, what if we have a situation where we have an increase in urea? So, if a patient has an increase in urea generation, even though the plasma osmolality increases to 315 milliosmoles per kilo, just as it did with our patient who has an increase in glucose, urea can cross the membrane freely. 05:30 Therefore, it distributes equally between the intracellular and extracellular compartment, and thus does not exert a tonic effect on water. 05:39 Alright, let's also review some of the osmolar substances within our body fluid compartments. So, in this graphical or in this table, what I want you to pay attention to is our top three labels, which include our intravascular fluid compartment, the interstitial fluid compartment, and then the intracellular compartment. 05:59 Now, both the intravascular and the interstitial fluid compartment make up our extracellular fluid volume. 06:05 I want you to pay attention to a few things just to keep in mind. 06:08 Let's look at sodium. Sodium, if you note, is primarily located within the extracellular fluid compartment. 06:15 So, highest in the intravascular volume at 142 milliosmoles per liter. 06:20 Potassium on the other hand is highest in the intracellular compartment. 06:25 And we only have very small amounts of potassium located in our extracellular fluid compartment. 06:31 Urea on the other hand is equally distributed among all depart -- all compartments. 06:37 And then, finally, when we look at the total last osmolar concentration, it's equal across the intravascular, interstitial, and the intracellular compartment. 06:47 So, when we think about water balance, we have to also think about something called obligate osmolar excretion. 06:56 This is how we can handle water. 06:58 So, obligate osmolar excretion is the amount of osmols that needs to be removed by the kidney in order to maintain osmolar homeostasis. 07:07 It's dependent on dietary intake. 07:10 So, our basal metabolism in the fasting state, we can generate about seven milliosmoles per kilo per day. 07:17 Now, our urine can actually be diluted to about 50 milliosmoles per liter, and it can be as concentrated as 1000 milliosmoles per liter. 07:29 So, if we either wanna get rid of free water or we wanna conserve free water, we can do that through manipulating the concentration of osmolarity in our urine. 07:38 This way, we can accomplish both osmolar and water balance simultaneously.
About the Lecture
The lecture Body Water Balance: Terms and Definitions by Amy Sussman, MD is from the course Water Balance: Hypo- and Hypernatremia.
Included Quiz Questions
What is the volume of interstitial fluid in a 60 kg (132 Ib) woman?
- 7.5 L
- 15 L
- 2.5 L
- 10 L
- 30 L
What is the primary determinant of plasma osmolality?
- Sodium
- Potassium
- Urea
- Glucose
- Proteins
Which of the following is true regarding plasma tonicity?
- It reflects the concentration of solutes that do not easily cross cell membranes.
- It does not affect the distribution of water between intracellular and extracellular compartments.
- Urea is a major determinant of plasma tonicity because it is equally distributed among all fluid compartments.
- It is the difference in the total osmolality between intracellular and extracellular compartments.
What is the definition of obligate osmolar excretion?
- The number of osmoles that need to be removed by the kidney in order to maintain osmotic homeostasis
- The volume of plasma that is completely cleared of a specific compound per unit of time
- The volume of fluid filtered from the renal glomerular capillaries into the Bowman capsule per unit of time
- The number of osmoles of solute per liter of solution
- The number of osmoles of solute per kilogram of solvent
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
5 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |