Playlist
Show Playlist
Hide Playlist
Types of IV Fluids (Intravenous Fluids): Saline: Clinical Terminology
-
Slides TypesofIVFluids RenalPathology.pdf
-
Download Lecture Overview
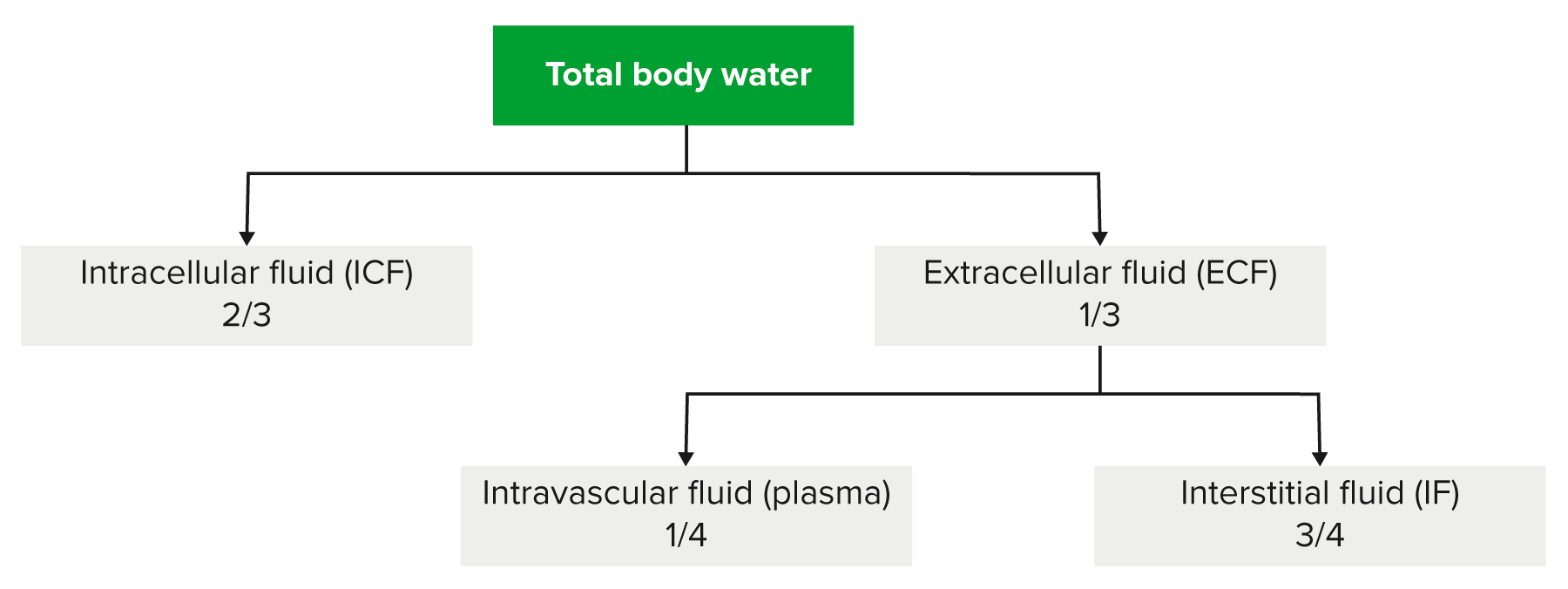
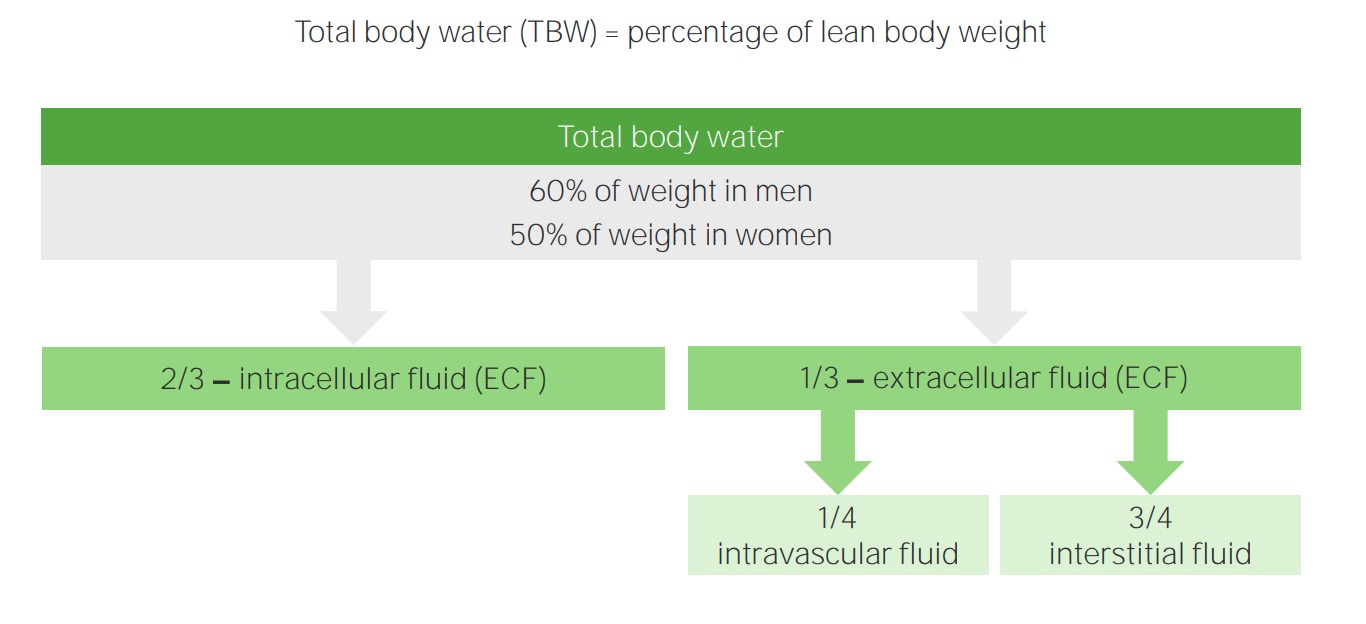
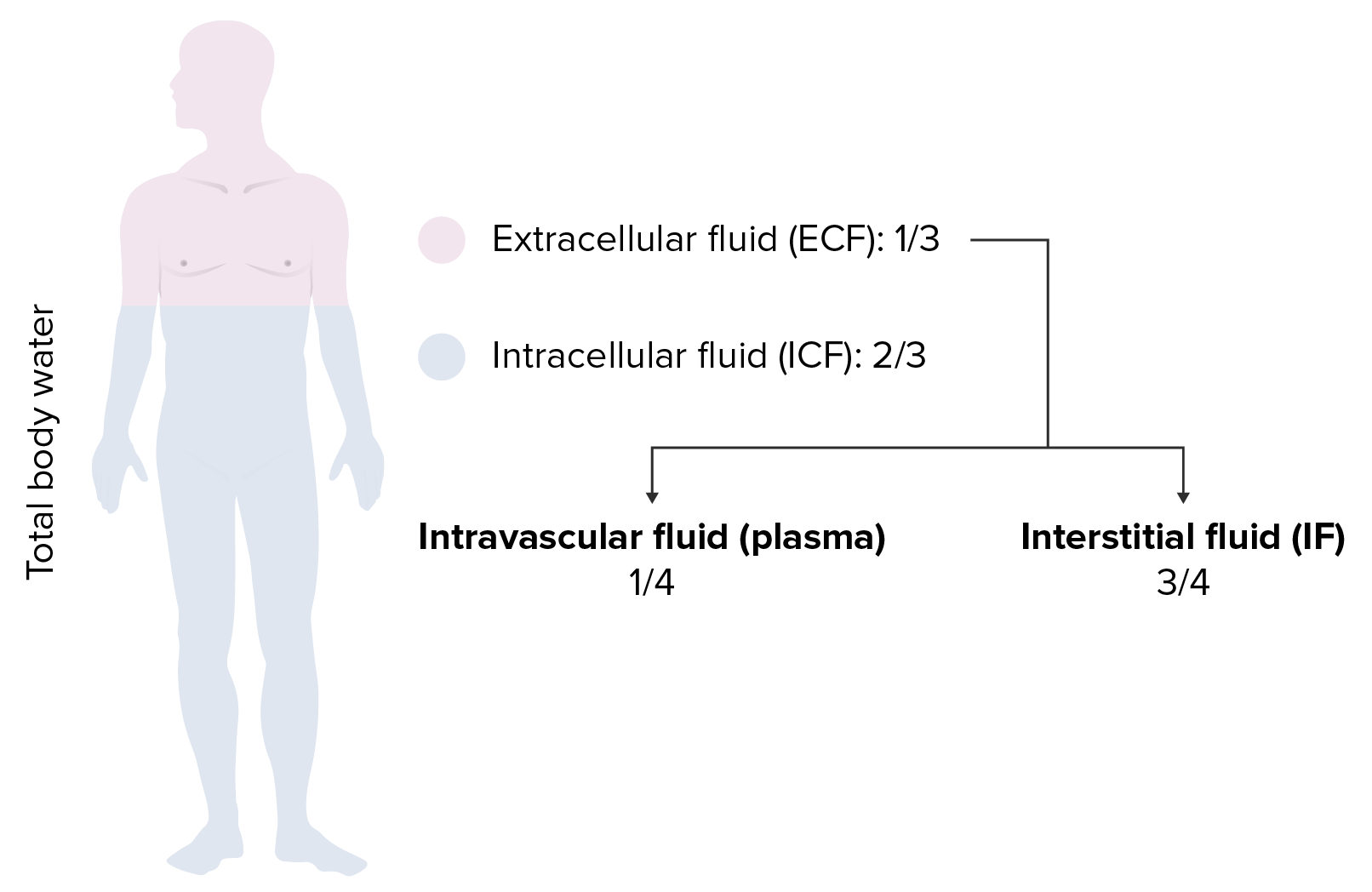
00:02 Saline is where we are and going to little bit technicality, but I am just going to give you a brief little overview of saline without going to enough detail where number 1 is irrelevant. 00:14 Anything is irrelevant at this point. It is not even included in any of my lecture series. 00:21 You understand that. Everything that I have been giving you is high yield. So, therefore, you don't hear me say high yield because there would be a waste of your time. Everything is high yield. Everything that I am giving you is a story about something significant in some way, shape or form. It is going to be asked. I can guarantee you that. 00:41 Okay. So come with the saline. It is NaCl that much we do know. Next, it is isotonic. 00:50 Now, what is interesting about isotonic is that you look at this and you see 154 and right off the bat, you will know that does not mean isotonic. Isotonic means approximately how much? Approximately 300. So what does this 154 then refer to? Each electrolyte, Na cation, chloride, anion, each electrolyte has a mmol/L unit of 154. 01:19 But Dr. Raj, if there is two of them and each one is 154, don't you get 308? That is correct. So technically speaking you are telling me Dr. Raj that therefore saline NaCl at 308 is slightly hypertonic. Yes, that is exactly what I am saying. Nothing is ever perfect, but this is as close to isotonic saline as you can get. We have therefore 0.9 percent. So it is not 100 percent that you would be using for being isotonic for obvious reasons. It really is a little bit more hypertonic. A 0.9 percent that is the calculation that you would use or shall I say 0.9 percent is the value that you want to know as it being isotonic. Let me give you another example. We have talked about the following. You have injected your patient with isotonic saline slowly over a course of 24 hour period. 02:13 If you by chance give too much NaCl, then you are filling up the entire ECF, you might then be in a state of hypervolemia. What happens to ECF volume? It increases. Is there any other change, yes or no in any of the total body water compartments? No. The only change was what? ECF volume. No change in osmolarity either in the ECF or ICF. That we have discussed. 02:42 If you want a little bit more detail in terms of the litres and the units and such, there it is at this point you are good with knowing 0.9 percent. Okay now, this discussion is important. I need you to understand the terminology of half normal saline. So if one half is normal saline, what is saline? NaCl okay. Saline is NaCl. So if half of your solution is NaCl, saline, what is the other half? Water. What is the difference in terms of distribution? Before we move on, let us now predict what you can expect on predict as far as distribution of the fluids. If it is going to be half normal saline, tell me about that saline. That saline will only be distributed in which compartment? I am sorry, what did you say? Only in the ECF, good. So the saline component of this is only going to be distributed in the ECF. 03:47 Tell me what are the fractions that you want to know. 3/4, 1/4. 3/4 interstitium, 1/4 plasma. Stop there. What about the other half? The other half was pure water. Okay. That pure water, tell me about its distribution throughout the total body water. Throughout, what does that mean? That means 1/3 of water will go into where? Good. It will go into ECF. 2/3 of it will go into the ICF and then from there, that 1/3 you will divide it into 3/4 and 1/4. 04:21 Is this too much detail? No. This is expected of you as being a fresher medical school student or a resident. Ya. Ultimately how often would you use IV fluids? All the time. So it is imperative that you at least know a few differences here and what it truly means to be normal saline versus half-normal saline. And if it is half normal, what is half of nine? There you go. 04:47 Take a look. 0.45. Why I say 9? Because normal is what? 0.9. Our thing is coming together. 04:55 Before we move on, one last thing. If each electrolyte had a millimole of 154 and you taken half of it, then this thing brings us to 77. Seventy-seven is designated for each electrolyte. Thus when you add them both, how much do you get? 154. Is 154 normal plasma osmolarity? Yes or no? Not at all. What is normal plasma osmolarity? Approximately 300. 05:26 So what kind of solution is half-normal saline? It is hypotonic fluid. Is it not, conceptually? So things that you want to keep in mind. That was a little bit of math, a little bit of calculation, let's put things together and as we do so, I am going to walk you through the concepts. That is the most important thing. You as a clinician, you as a doctor are always thriving for concepts. Once you get the concepts down, then you can put in the math as you wish. But conceptually, one can think of the following, we have examples here. Let's do half normal saline. What does that mean to you? Half water, half saline. The half of the water, which is our first component here, what does it do? Look. Equally distributed through TBW. What is highlighted here in red? The total body water includes 2/3 of ICF, 1/3 of your ECF. What about the other half? The other half is saline. Where are you only going to distribute? Can you predict this? Good. There you have it, ECF. 3/4 interstitium, 1/4 plasma. Did you ever think that something that you learned way back in physio in terms of basic concepts of total body water, will become so incredibly crucial for you to understand the pathology? We have spent quite a bit of time with total body water compartments with all kinds of diseases and now we are finishing it up. We are adding the icing with IV fluids. Now, what is going to happen to the first quarter? What does that mean? The quarter is referring to the quarter saline. That would mean only 1/4 of this is saline and what about that 1/4? Now we can do a little bit math here, can't we? A litre of quarter normal saline. What is a litre? 1,000 mL. Clear. 07:18 Of the 1,000 mL, and I am then giving your patient quarter normal saline, how can you think of this? We will divide this into of the saline, you then take your free water, which is going to be 750. Because only a quarter of this is saline. 750 is what? You tell me about that water and distribution. You got this down? What about that water? Throughout total body water. ECF, ICF, all throughout it and what does that quarter mean? 250 would be your saline and tell me about that 250. Good. It will be distributed between your ECF only. This is what kind of fluid? Once again hypertonic. So now we have normal saline, half-normal saline, quarter normal saline. This differs down we have added a little bit of math with one litre of quarter normal. Let us continue.
About the Lecture
The lecture Types of IV Fluids (Intravenous Fluids): Saline: Clinical Terminology by Carlo Raj, MD is from the course Fluid and Electrolyte Balance.
Included Quiz Questions
How many mmol/L of Na⁺ is there in 0.9% normal saline?
- 154 mmol/L
- 308 mmol/L
- 100 mmol/L
- 50 mmol/L
- 77 mmol/L
From one liter of ‘half-normal’ saline, how many milliliters of free water would you expect, assuming the remainder is isotonic saline?
- 500 mL
- 1000 mL
- 200 mL
- 750 mL
- 250 mL
Within which fluid compartment would you expect the free water component of a hypotonic saline solution to distribute?
- Total body water
- Extracellular fluid compartment
- Interstitial fluid compartment
- Intracellular fluid compartment
- Plasma compartment only
What is the total osmolarity of ½ normal saline (0.45% NaCl)
- 154 mmol/L
- 308 mmol/L
- 100 mmol/L
- 50 mmol/L
- 77 mmol/L
Of one liter ''quarter normal'' saline, how many milliliters of free water would you expect, assuming the remainder is isotonic saline?
- 750 mL
- 500 mL
- 250 mL
- 100 mL
- 200 mL
From one liter of ‘quarter-normal’ saline, how many milliliters of isotonic saline would you expect, assuming the remainder is a free water?
- 250 mL
- 750 mL
- 500 mL
- 200 mL
- 100 mL
What is the total osmolarity of normal saline (0.9% NaCl)?
- 308 mmol/L
- 154 mmol/L
- 100 mmol/L
- 150 mmol/L
- 90 mmol/L
How many grams of NaCl is there in 1 liter of water?
- 9 g
- 10 g
- 15 g
- 20 g
- 8 g
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
1 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |
This was a good course because the lecturer gave examples and explanations.