Playlist
Show Playlist
Hide Playlist
pKa and Drug Solubility – Absorption and Distribution | Pharmacokinetics (PK)
-
Slides pKa and Drug Solubility Absorption and Distribution Pharmacokinetics PK.pdf
-
Download Lecture Overview
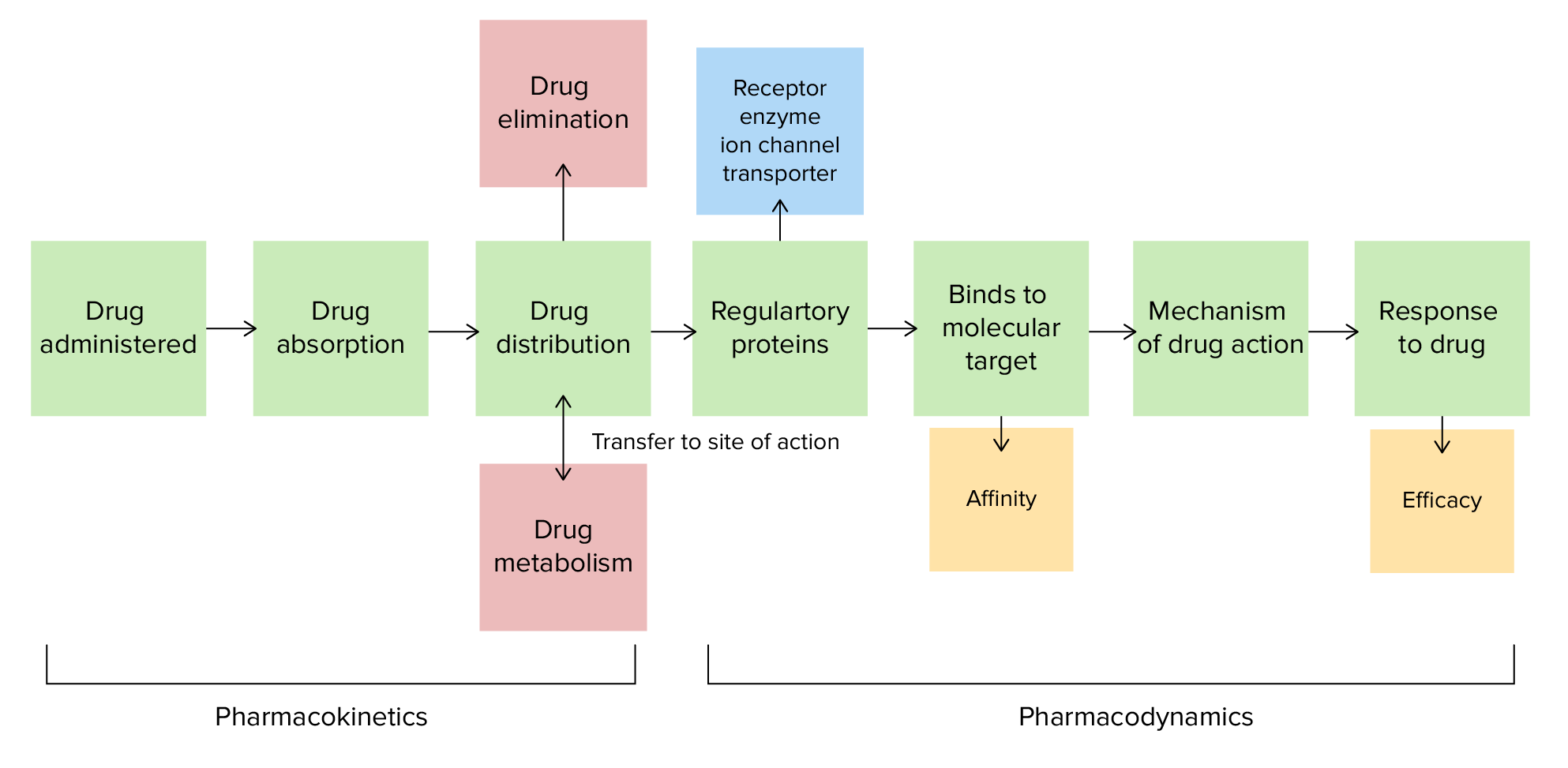
00:01 Okay. Let's talk about a new topic. Something called pKa. Now look at this question. Spend a little bit of time reading it. 00:10 And then we will try and go through the answer together. Okay so hopefully you have read the question. What I want to do is I want to focus a skill that you need for your exam. When you are writing an exam and you see a long stem like this especially for the USMLE exam. Look at the last sentence first. Because what that's going to do it's gonna focus your attention. Now in this particular question, the last sentence says that it has a pKa of 3.8 and what percentage of the drug will be hydrophobic. Well, all of a sudden you have realised that the first half of that question is completely useless to getting your answer. So you can actually just ignore a lot of that clinical information and get to the answer much quicker. 01:00 So now what we do is we want to pick between the answers. 100%, 50%, 10% and 1%. So what percentage of the drug will be hydrophobic at a pH of 4.8 if the pKa is 3.8. Well what is pKa? Let's go over that together. So, aspirin has a pKa of 3.8. So what is pKa? The pKa is that pH level at which a drug is 50% protonated and 50% non-protonated. So the ratio of protonated and non-protonated forms is 1:1. For weak acids and you can see that because the pKa is 3.8 which is less than 7, that's an acid. So, for all weak acids, the ratio changes to 1:10 or 10% at 1 pH unit more alkali than the pH. 01:55 And the ratio changes to 1:100 or 1% at 2 pH units. So remember that pH is a logarithmic. So 1 unit is 10, 2 units is a 100, 3 units is a 1000. So if you have a pKa of 3.8, and the pH of 5.8, you've got a pretty significant difference in ratio. So 2 pH units is 1:100. So let's take a look at pyrimethamine. Pyrimethamine is another drug that has a pKa of 7.42. That makes it a weak base, right. Because the 7.42 is actually higher than 7.0 so anything above 7.0 is a base. The pKa is the point at which a pH that gives you 50% protonated and 50% non-protonated drug so the ratio is 1:1. For weak acids and bases the ratio changes by 1:10 or 1:100 for each 1 or 2 pH units you are drug so the ratio is 1:1. For weak acids and bases the ratio changes by 1:10 or 1:100 for each 1 or 2 pH units you are For weak bases, the ratio changes to 10:1 at 1 pH unit more alkali than the pKa. 02:56 Now because it's a base, the more alkali the pH is it makes less soluble or less polar in the protonated form. 03:05 So to go over it again for weak bases, they are ionized. 03:10 They are more polar when protonated and more soluble when pronated. 03:15 With weak acids, they are not ionized. They are less polar when protonted and less soluble when protonated Let’s have a short look at the underlying mathematical behind this. 03:26 The Henderson-Hasselbach equation approximates the relationship between the pH of a solution, the dissociation constants (pKa or pKb) and the ratio of the concentrations of the dissociated chemical species at equilibrium. 03:42 You won’t need to know the details of these calculations but the ratios for nonprotonated and protonated forms of the drugs described earlier are derived from this equation. 03:53 So how did we get the ratio for weak acids? Again, this calculation is only for a better understanding and not mandatory. 03:59 We start with an equation for the acid dissociation constant at equillibrium, based on the concentrations of the chemical species, hydrogen ions, and conjugate base. 04:09 Then, we take the logarithms of both sides and reverse all the mathematical operators. 04:16 Since the negative logarithm of the concentration of hydrogen ions equals the pH and the negative logarithm of Ka equals pKa, we simply replace them in the equation. 04:28 Then, we can rearrange the equation and invert the numerator and denominator to get the Henderson-Hasselbach equation for a weak acid. 04:38 Lastly, we take the antilog of each side. 04:41 Now you know how we have a formula to determine the ratio of chemical species based on the pH and pKa. 04:49 Let’s take aspirin, a weak acid, as an example, which has a pKa of 3.8. 04:54 All we have to do at this point is plug in values into the formula. With a pH of 3.8 and a pKa of 3.8, we get 10 to the power of 3.8 minus 3.8. 05:04 So, the ratio here is 1 to 1, 50 percent ionized and 50 percent not-ionized. 05:11 Now, let's say there is a pH of 4.8. Plug this into the equation, while the pKa remains 3.8. 05:17 10 to the power of 4.8 minus 3.8. Our ratio is 10 to 1. This means it’s 90 percent ionized and 10 percent not. 05:25 Lastly, at a pH of 5.8, we get a ratio of 100 to 1, so 99 percent is ionized. 05:33 Keep in mind that weak acids and bases have pKa’s between 2 and 12. 05:37 Strong acids and bases dissociate almost completely, so 0 percent remains. Thus, the Henderson-Hasselbach equation does not apply. Also note, that pKa plus pKb is always 14. 05:51 Now let’s look at it for a weakly basic drug. 05:53 The ionization constant at equilibrium here is Kb, and the concentrations refer to the base, hydroxide, and the base's conjugate acid. 06:04 First, we convert to the acid dissociation constant, Ka, by using what we know about Kw (the constant for water). 06:13 Remember Kw = Ka times Kb. 06:16 Then, we take the logarithms of both sides and reverse the signs. 06:21 Just like before, with the weakly acid drugs, pH and and pKa are substituted in. 06:27 Now we reverse signs and rearrange the equation again. 06:31 In the last step we reverse the sign of the logarithmic fraction once again by inverting the numerator and denominator. This brings us to the Henderson-Hasselbach equation for a weak base. 06:42 The proportions of a weak base in non-ionized and ionized forms at any pH can therefore be written like this. 06:49 Lastly take the antilog of each side and we end up with a ratio of unionized and ionized forms equal to 10 to the power of the pH minus the pKa. 06:59 Let’s try it with pyrimethamine, a weak base, with a pKa of 7.4 With a pH of 7.4 our equation looks like this, resulting in a 1 to 1 ratio. 07:10 Thus 50 percent is non-ionized and 50 percent ionized. 07:14 A pH of 8.4 leaves us with a ratio of 10 to 1, meaning that 90 percent is non-ionized, and 10 percent is ionized. 07:23 Lastly, for a pH of 9.4, we get a ratio of 100 to 1. I think you got it now. 07:28 So speaking of questions, let's go back to our question and our case. The ratio changes from 1:1 to 1:10 at 1 pH unit more alkaline than the pKa. 07:39 And it changes to a ratio of 1:100 at 2 pH units that are more alkaline than the pKa. So in the case of this question, we were saying the bottle label says that the substance has a pKa of 3.8. What percentage of the drug will be hydrophobic or lipid soluble or lipophilic in the small bowel at a pH of 4.8. Well what's the answer? So the answer is C, 10%. 08:08 1 pH unit difference, 10%. 1:10 ratio. Now, why do you care about a pKa? This sounds like a lot of chemistry that you left behind in pre-med. Well the reason why we need to know this is because we need to know how to enhance excretion of a toxin. 08:42 So for example in aspirin overdose we want to alkalinize the urine. When we alkalinize the urine with sodium bicarbonate we trap the aspirin molecules in the urine so it can't get reabsorbed back into the body. That's how we treat an aspirin overdose. 08:59 It's also useful when we want to design a drug and when we want to mix medications. So in the pharmacy world, it's also something that's very important.
About the Lecture
The lecture pKa and Drug Solubility – Absorption and Distribution | Pharmacokinetics (PK) by Pravin Shukle, MD is from the course Pharmacokinetics and Pharmacodynamics.
Included Quiz Questions
A 44-year-old woman presents to the hospital ER with a low blood pressure of 77/40, a heart rate of 40, and normal pupils. She has an empty bottle of blood pressure pills that have a pKa of 7.5. You want the patient to excrete the drug in the urine. What is the most appropriate action?
- Acidify the urine. This will protonate the drug, allowing it to be more soluble in the urine.
- Alkalinize the urine. This will protonate the drug, allowing it to be more soluble in the urine.
- Acidify the urine. This will deprotonate the drug, allowing it to be more soluble in the urine.
- Alkalinize the urine. This will deprotonate the drug, allowing it to be more soluble in the urine.
- Do not change the acidity of the urine.
A 5-year-old child overdoses on her mother's anti-depression medication. The pKa of the drug is 3.9. The pH of the small bowel is 5.9. What is the ratio of protonated to unprotonated molecules?
- 1:100
- 1:10
- 10:1
- 1:1
- 100:1
A patient has overdosed on pyrimethamine, which has a pKa of 7.42. What percentage of the drug will be trapped in the urine if the urine pH is 5.42?
- 99%
- 90%
- 9%
- 1%
- .9%
A patient has overdosed on pyrimethamine (a weak base). It has a pKa of 7.42. What portion of the drug will be hydrophilic at a urine pH of 8.42?
- 10%
- 90%
- 5%
- 1%
- 99%
Customer reviews
3,2 of 5 stars
| 5 Stars |
|
4 |
| 4 Stars |
|
1 |
| 3 Stars |
|
0 |
| 2 Stars |
|
1 |
| 1 Star |
|
3 |
the lesson is properly given, but requires a previous base, which must be checked back in books, even that is a useful video and the content is clear enough .
Rather than giving us mnemonic, I'd rather had him explained. I had to go to Youtube to find out what this means and it took me a lot of time.
I love the course, it's easy to learn and to understand
sorry to say it is really not well explained or concept clearing kindly modify lecture