Playlist
Show Playlist
Hide Playlist
Extracellular Fluid (ECF) and Intracellular Fluid (ICF): Plasma Osmolality (POsm)
-
Slides WaterandSodiumPathophysiology RenalPathology.pdf
-
Download Lecture Overview
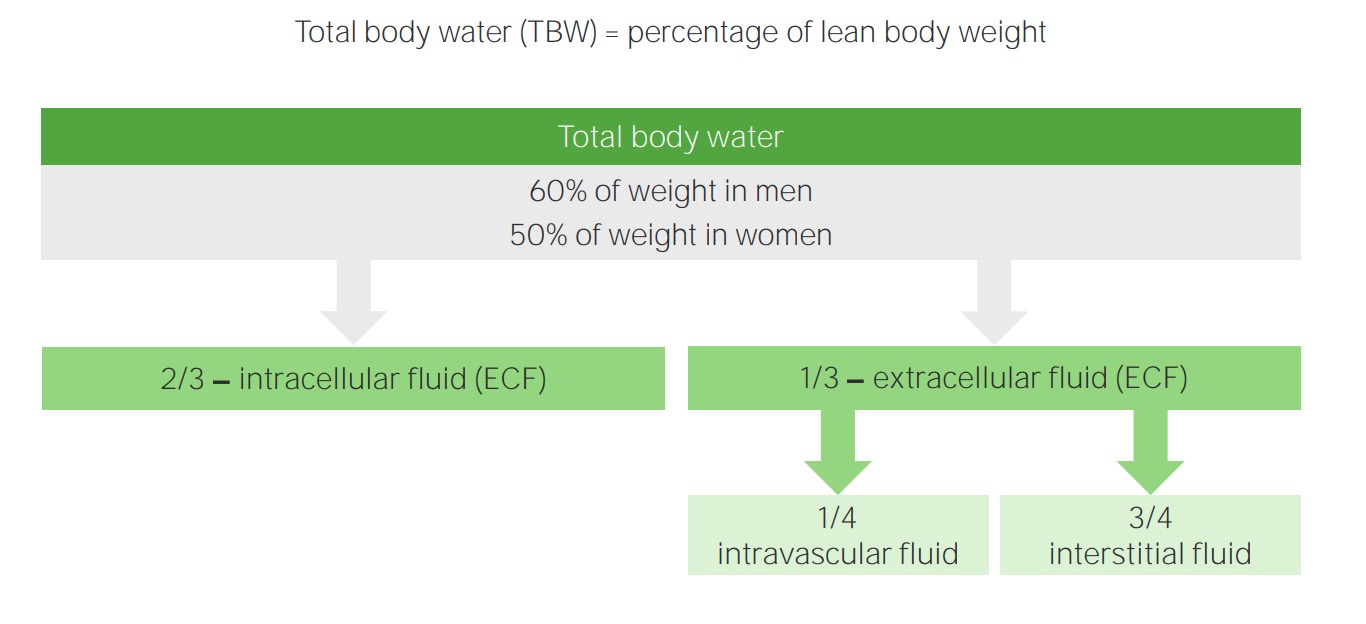
00:01 Plasma osmolality, what is osmolality? Osmolality is the number of solutes in plasma. In other words, we are talking about the tonicity in ECF and by that once again you know me now. 00:13 I am not just going to read to you. I am going to have you conceptualize. In the ECF, what is the most important compartment that we're monitoring please? What is that? Good. That is the plasma compartment and in terms of actual fractions, what is it? A measly 1/4, use that you will be fine. 1/4, quarter that is which you are measuring in terms of tonicity. Next what does isotonic mean? Normal plasma osmolality. To make things simple, we will keep it around 300 okay. Now, of course, you want to be technical later on, which is between 275 and 295 and then I will give something dramatic. If they want you to know the plasma osmolality has decreased too much, then I will give something like 250 to 245. What do you mean they? Well the patient or maybe perhaps a question. And if it is too much, let it above 300. Hypotonic what does that mean? Where am I right now? In the plasma okay. The plasma compartment and you find that they have a hypotonic state. What does that mean to you? What does that mean? It means that things are diluted right. Now, what does that mean to you in terms of plasma osmolality? You should know you are less than 275 maybe at 260s. You're on the lower end of your plasma osmolality. Keep that in mind because what we are going to do? We are going to walk through this and then well we are going to have some important differentials. Okay. By the time we are done with the section, all this entire lecture series of water and sodium pathophysiology, we will have gone through the details of SIADH, diabetes insipidus and we will have gone through psychogenic polydipsia. Okay. So these are the three that always come into mind and these are the ones that students tend to confuse because they are not well. You are not going to do this anymore. I am going to show you how to think through this and know as to what you are paying attention to so that you never miss the question. Hypertonic state. What does that mean to you? That means that you have too much "solute." Maybe too much sodium inside my what compartment? Good. Plasma compartment. 02:11 What does all this mean? You will see in a little bit. Okay. An important formula that you want to keep in mind now. I am going to walk you through which you need to know for physio and then of course what we need to know in pathology. The most important component that is then going to really dictate or determine your osmolality is your sodium. But going back to our initial statement in our philosophy of electroneutrality, what does that mean? Give a cation such as sodium and then with it, it has to be accompanied by a chloride. 02:46 So therefore if I tell you the sodium is the most important component to determining then tonicity or plasma osmolality, then you must take what into consideration? The chloride So then one becomes two. What does that mean? Two times sodium will give you proper amount of plasma osmolality physiologically okay. Physiologically, take a look at the second component, glucose. Tell me about glucose. We will take a look at the formula, a serum glucose over or divided by 18. So normally speaking if you have plasma glucose approximately how much, please? Everything that you do at some point you have to be able to interpret your labs. So I am going to hammer home glucose once again. Keep it simple at 100. 100/18 gives you a very small number physiologically and so, therefore, the glucose at this point could be negligible. Remember you're clinician. Well if you want to research in such, that is on your own time, isn't it? Our first priority is to make sure that that patient is surviving, is maintained and properly managed. So what are you looking for here is two times sodium physiologically. The glucose at this point can be ignored. 04:02 But when does the glucose come into play? What does diabetic ketoacidosis mean to you? Diabetic ketoacidosis. Absolute uncontrolled diabetes mellitus. Uncontrolled. Most likely occurs in which diabetes type? Diabetes mellitus type I, why? Because this was a child that wasn't born or should I say that the insulin levels pretty much got exhausted immediately very quickly and so therefore if there isn’t proper control and there is no insulin, what is going to happen to that glucose level in that patient? 400, 500, 600, 700, 800, extremely high. Now this glucose becomes an important factor in plasma osmolality. Of course, it does. You go as far as that. You will be fine. The physio part, two times sodium, you add in the pathology such as diabetic ketoacidosis, you will have to take glucose into consideration and we will repeat this entire concept again in the Darrow Yannet box. What about urea? You should know urea and if you don't please pay attention. Urea, extremely unpredictable. 05:16 Urea in the body physiologically is not at all ever going to contribute to plasma osmolality. 05:25 Technically it is a part of the equation. Yes, it is. Let it go. I beg of you. Time is too precious. Now technical plasma osmolality is between 275 to 295 and what is plasma osmolality? It gives you an approximate correlation with the amount of sodium concentration. 05:44 Plasma osmolality, well here we go but urea I just want to focus and make sure that you understand that urea is too unpredictable. So mean to say at times it is just going to shift back and forth between ECF and ICF. It does not contribute to effective osmolality. Nephrologists, as I said once again as far as effective osmolality is urea is not going to contribute to. What are the two major things? Sodium, glucose when? If your patient has hyperglycemia. That is the equation ladies and gentleman that you are focusing upon. Here we take a look at sodium and glucose and its totality of ECF and what it means to be impermeant, shall we? Okay. What I'd like for you to do on this slide is really be able to conceptualize what I wish for you to see not in terms of the verbiage here but what is actually taking place in your body. And in my opinion one of the most effective pictures that you can create for yourself would be the Darrow Yannet box so that you can see in a very organized fashion as to other shift of fluid between ECF and ICF is taking place. So with that said, that will return a complex with the slide. Begin with sodium and glucose. First and foremost once again go back to your basis of total body water. Work with me here. Simple questions but yet also every effective and this is the kind of stuff, this is the platform that requires in which you will build and build again into pathology and you are just going to walk through the stuff and you will feel good. You really will on every single level and you won't be doubting yourself. Don't you hate that feeling of just doubting? Did I say that right? Did I come across right? Did I chose the right answer? You will be confident. So what is sodium and glucose? For total body water, there are two major barriers in the total body water system. The first barrier is going to be between the ECF and ICF. What is the name of that membrane or that would be a simple question. That membrane well, let me give you two choices. Is it the cell membrane or is it the capillary membrane? That is your cell membrane, isn't it? Between what? ICF and ECF. It has to be the cell membrane. That cell membrane now read impermeant to whom? Sodium and glucose. Are we clear? That is one membrane, but Dr. Raj, you said there were two. I did. So where is the second one? The second one actually is in the ECF, isn't it? So with the ECF, what two compartments are there again? The most important compartment is which one at all times physiologically? Plasma compartment, isn't it? In terms of fraction, what are you going to use? 1/4. Clinically understand that it is approximately 1/3. Let us go with what is most commonly asked, 1/4, is your plasma. What is the name of that barrier membrane that seperates the plasma from the interstitium? That would then be your capillary membrane. Good. Now that capillary membrane is permeant to sodium, isn't it? Yes, it is. That pitting edema that we talked about so many different times, does that contain sodium? Always. Yes, it does. Transudate, the pitting edema, which is protein poor, sodium rich. Protein poor, sodium rich equals transudate. 09:23 So that means the sodium did pass through the capillary membrane that has to be cleared. 09:29 Now, let us move on. 09:31 Changes in the concentration will produce an osmotic gradient obviously. Now these are the steps that you will be taking chronologically, forever more clinical practice, any board exam in which you will get every single question right as long as you follow this order of pattern of the fluid shift. When you urinate too much, for example diabetes insipidus, let us say uncontrolled diabetes mellitus, doesn't matter. Both of those have what? Polyuria. 09:58 Did you just hear what I just said? Polyuria. You hear that. You automatically think actually three differentials for the two important ones at this juncture. Both begin with the prefix diabetes. One is insipidus, one is uncontrolled diabetes mellitus correct. Okay. 10:17 My point is this. If you are urinating in great amounts, then what happens to your plasma compartment? It is going to decrease. Is that part of ECF? Yes, it is. So you tell me what kind of change is now taking place with the ECF compartment? A decreased in ECF volume, number 1. And as we continue through here, we are going to add more chronological changes so that you eventually come to understand what kind of shift we have between the two compartments of ECF and ICF? That shift between ECF and ICF is controlled by whom? The osmolality. 10:54 Now, this brings us to number 2 or step number 2. While step number 1, when you are losing fluid, ECF volume. Now the opposite could be held if you are putting in too much infusion of saline, then you would have increased in ECF volume. Anyhow, your first step is going to be ECF volume either a diminished effect or an increased effect, what's the second step? It is the osmolality. 11:21 In diabetes insipidus as an example, if you are losing too much fluid, ECF volume decreased number 1, what happens to ECF osmolality? Increases. Are we clear? Because you are losing fluid. 11:30 That is important. Step number 2, let us move on. Now the water moves from obviously there is osmosis here and you tell me. There is an increase in ECF osmolality. What step is this? Number 2, tell me about this fluid shift. Water is being pulled out of the cell. This is due to what? This is due to that osmosis. So in your head clinically step number 3 would be ICF volume and in diabetes insipidus that ICF volume is then going to be decreased. Clinically this then explains what we just talked about water moving from a low to high solute concentration. 12:16 Now here we have water shifts. Do not alter with urea concentration. As I told you earlier and I emphasized this with our discussion of urea. With urea, it is not part of your effective osmolality in clinical situations. Is that understood? So what are the two effective osmolalities? It is the sodium times 2, why? Because it is always attached to chloride and you will take glucose into consideration if you have uncontrolled diabetes.
About the Lecture
The lecture Extracellular Fluid (ECF) and Intracellular Fluid (ICF): Plasma Osmolality (POsm) by Carlo Raj, MD is from the course Fluid and Electrolyte Balance.
Included Quiz Questions
Why is sodium doubled in the plasma osmolality equation?
- In order to account for electroneutrality
- Because it travels as a diatomic molecule
- It is the proportion of Na compared to other solutes
- It is related to the atomic weight of sodium
- It is twice the size of the solvent molecule
Urea is NOT considered relevant for effective osmolality but it IS relevant in the maintenance of the countercurrent mechanism. Which of the following explains this dichotomy?
- All are correct
- Thick ascending limb is impermeable to water
- Urea diffuses freely between ECF and ICF
- The capillary membrane is permeable to water and solute.
- The cell membrane is permeable to water.
Why is urea ignored when nephrologists discuss effective plasma osmolality?
- Water shifts do not occur with a change in urea concentration.
- Urea is highly polarized.
- All answers are correct.
- Urea is limited by renal blood flow.
- Urea complexes with albumin.
Which of the following statements about cell membrane permeability is correct?
- Fluid moves according to the osmolality.
- It is impermeable to urea.
- It is permeable to glucose.
- It is permeable to sodium.
Which of the following fluid compartment changes would you expect to find in a patient with diabetes insipidus?
- Increased ECF osmolality
- Increased plasma volume
- Increased ICF volume
- Decreased ECF osmolality
- Increased ECF volume
Which of the following statements about fluid compartments is INCORRECT?
- The capillary membrane is impermeable to sodium.
- Shifts between the ICF and ECF are controlled by solute concentration.
- The cell membrane is impermeable to sodium and glucose.
- The plasma compartment and interstitium are separated by the capillary membrane.
- ECF and ICF are separated by the cell membrane.
Customer reviews
4,7 of 5 stars
| 5 Stars |
|
2 |
| 4 Stars |
|
1 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |
This guy is awesome!, clear and straight forward. I love the way he motivates me to just keep the important data and let go unnecessary details.
Best lecture. I like the video.I read the slide with this lecture that help me to understand the our lecture.
I understand, but I think, the lesson needs drawings and diagrams, but is very well, thanks