Playlist
Show Playlist
Hide Playlist
Types of Anemia – Red Cell Disorders
-
Slides Disorders of red cells.pdf
-
Download Lecture Overview
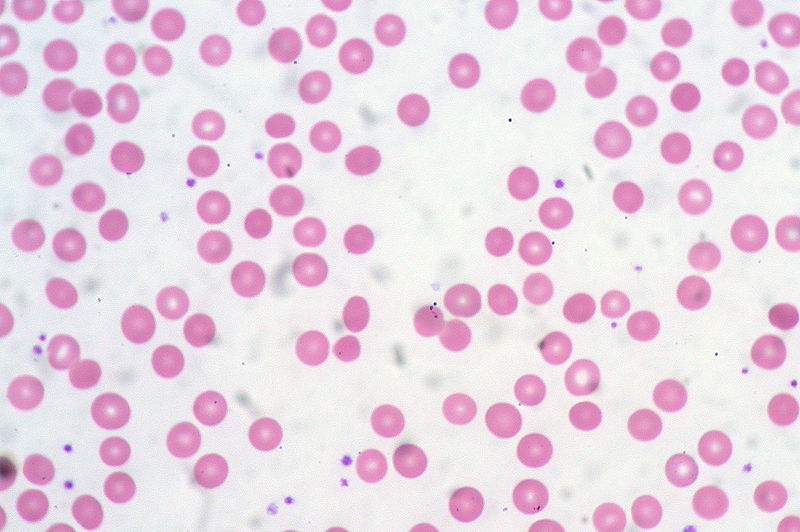
00:00 Now let's look at the range of diseases in which the anemia is associated with red cells of a normal size normocytic anemia. One of the most common that we see in hospitals now is the anemia of chronic disease, the anemia of chronic inflammation. This is a very interesting background. Whenever there is chronic inflammation or infection within our body, the body sequesters ion within macrophages. It starts to shut down the flow of ion within the body. 00:37 Now this leads to a reduced ion level in the blood and the reduction in hemopoiesis. Now you might ask "Why on earth does the body do this?" Well it's almost certainly a physiological evolutionary selected mechanism to limit the amount of ion available for pathogens like bacteria. Ion helps bacteria divide and here is a mechanism of hiding it away from bacteria. 01:08 There are 2 pictures on the right showing examples of how this may happen. At the top, we have a patient with rheumatoid arthritis and chronic disorders such as that are often associated with some form of mild anemia. And at the bottom, you'll see a bone marrow stain for ion and the blue there represents the ion which is present within the reticular endothelial cells. It's being taken in because of anemia of chronic disease. It's quite a nice illustration of how ion really has to be very carefully regulated in our body. Now the treatment of anemia of chronic disease is really to treat the underlying condition. Treat the rheumatoid arthritis or perhaps the malignant disease or whatever it may be is leading to this condition and then the rest of the anemia will sort itself out. Another important cause of a normocytic anemia is renal disease. Because remember that the kidney produces erythropoietin, the major regulator of erythropoiesis. You'll see on the right a chart showing this very beautiful feedback mechanism for regulating erythropoiesis. In the bottom, the kidney acting as an oxygen sensor and producing erythropoietin whenever there is hypoxia releasing that which leads to increased erythropoiesis and the correction in the red cell mass. If the kidney is damaged, it may not be able to make erythropoietin and anemia used to be a very severe problem in patients with chronic kidney disease. Unfortunately, we know a lot about erythropoietin though and it's being cloned and can be produced as molecule for injection was widely used to patients with chronic renal failure and helps to increase their hemoglobin. 03:15 Another time where you may see a normocytic anemia is when the bone marrow itself is involved through other diseases. Now in this situation, you'll also see reduction in white cells and platelets. The bone marrow simply can't produce enough normal cells. This can be due to a wide range of clinical problems. I've listed some there. Infiltration with leukemia, myeloproliferative disease, infiltration with carcinoma, or aplastic anemia. On the right, you'll see a trephine biopsy of the bone marrow and I don't know if you can recognize yourself, this is a difficult slide of what that may be, but in fact that pink tissue is metastatic carcinoma and that was leading to anemia in this patient. Let's now turn to macrocytic anemia where you have large red cells. The major cause here is a disorder called megaloblastic anemia. Quite a long word again but we can easily understand it; megalo large, blast the primitive erythroblasts. Look at the right hand side and you'll see those very large purple erythroblasts within the bone marrow. Why are they large? Well, essentially the vitamin deficiency here is limiting their ability to divide and replicate so they're trying to expand and produce more molecules ready for division but they simply don't make the final division. This is due to deficiency of vitamin B12 with folate. A characteristic feature within the blood film is shown at the bottom and you'll see 2 white cells which you will recognize as neutrophils. Now neutrophils have multilobed nuclei and you will see there more lobes than you would expect normally up to 5 but in this condition you can see more than that, characteristic feature of megaloblastic anemia. Now let's talk in a little bit more detail about how this megaloblastic anemia can arrive. The most important course that you need to know about is pernicious anemia. This is now recognized as an autoimmune disease in which the patient makes antibodies to the stomach to gastric parietal cells and to a protein called intrinsic factor which is made within those cells. Now remarkably, when B12 is absorbed within our food, it has to bind to intrinsic factor. The complex of B12 and intrinsic factor is then absorbed in the terminal ileum. You can see immediately but if your intrinsic factor is neutralized with antibodies, you won't be able to absorb B12 and I have mentioned there that neuropathy can develop and it's certainly true that this disorder can lead to numbness in peripheral nerves and if untreated which thankfully we exceptionally rarely see these days, it can lead to very severe problems even blindness or dementia. I should mention that before we knew the cause of pernicious anemia, this was a major cause of death of patients over 100 years ago. Treatment is very simple, vitamin B12 injections given every 3 months. You can also take high doses of oral pure B12 and hoped that you've just manage to absorb enough. Now the bottom, I've mentioned folate deficiency. This is not an autoimmune disease, but it can also occur when there are excess requirements for folate classically when somebody's pregnant or if they have a hemolytic anemia and here again a simple tablet of folic acid will prevent all of their problems. When folate is used in the same biochemical pathway as B12 which is why these 2 cause the same effect of megaloblastic anemia. Let me now turn to another form of anemia, hemolytic anemia. This is a very interesting condition and we remember that red cells normally live for around 120 days, but if this is short term due to hemolysis, the structure of red cells, then the bone marrow must response to that by producing increased numbers of red cells. Interestingly, reticulocytes are slightly larger than red cells. So in fact the mean cell volume within the blood increases. So this anemia can be macrocytic in some cases. Now the bone marrow can normally cope with a shorter life span of red cells until it goes below around 15 days and then really it just cannot keep up and anemia is almost inevitable and that is hemolytic anemia. Let's look at how that can present in a patient. Let's start with that figure on the right of somebody who, I think you will agree, is jaundiced. You can see the yellowness in the sclera of the eye. That is bilirubin. Why on earth would somebody with hemolytic anemia become jaundiced. To understand that, we have to think about how hemoglobin is degraded. 09:10 We talked earlier about how it synthesized and you'll see in the diagram on the left how this occurs. Degradation of hemoglobin involves firstly the split into heme and globin. The globin is a protein and that is broken down into constituent amino acids. We are more interested in the heme. Again, the ion has to come out and it's recycled, but what's left is protoporphyrin and that's broken down into bilirubin and now you can see why patients may get jaundiced. 09:50 That bilirubin is insoluble, it needs to bind to albumin and it goes to the liver where it can be conjugated and excreted into the gut. Some of it is actually reabsorbed and can be passed out in urine. Now hemolytic anemias should be classified into 2 large groups. Disorders which arise because of an inherited abnormality and those which required later in life. Now red cells are relatively simple cells as cells grow. They have a membrane, they have hemoglobin, and they have some enzyme and indeed inherited courses of hemolytic anemia can involve those 3 major components. Let's look at those 3 in turn. We now need to just look quickly at the red cell cytoskeleton because of course the shape of a red cell is defined by proteins within the cell that maintain its structure and you'll see this cross section of a red cell, at the bottom we've got proteins called spectrin which form a lattice within the cell. And spectrin is anchored to the surface of the red cell to anchoring proteins and proteins such as bam 3 and bam 4.1. So these are very critical proteins for maintaining red cell shape. But what happens if you're born with an abnormality in the genes coding for some of these proteins such as spectrin or ankyrin. Well one of those disorders is hereditary spherocytosis. Have a look at that film on the right hand side. Do you see anything unusual about it? Well I think what you might see is some cells which are very round and darker than normal red cells, very spherical. 12:00 And those are the cells of hereditary spherocytosis. Now, these patients often are mildly anemic. These are very variable disease depending on the severity of the mutation and how it affects the individual patient. Folic acid can be useful to maintain and support red cell production and if the patient is getting symptomatically anemic with a low hemoglobin, then you can remove the spleen and splenectomy will solve the problem. It doesn't change the genetic defect but it stops the spleen from taking out these slightly different spherical red cells. But we have to be careful because there are risks associated with taking out the spleen particularly in children and you have to balance the anemia against the slight but definite risk of infection following splenectomy. There are other examples of inherited abnormalities of the red cell membrane, one of those I've listed as hereditary elliptocytosis and yes you're right, the red cells do look elliptical in that disorder. Let's look at another inherited abnormality of the enzymes this time. One of the important ones is glucose 6-phosphate dehydrogenase, long word, and this is more common in people from the Mediterranean and African region. Why? Because a heterozygous disorder can provide some protection against severe malarial infection. Now this is excellent and patients need to avoid factors that precipitate crisis of acute anemia in this condition. Sometimes it's drugs bizarrely, sometimes it's kidney beans, fava beans, and that can trigger a crisis. Just look on the right and you'll see that coming in from the right is what we call oxidant stress. If drugs, infection of kidney beans lead to oxidation within the red cells, the lack of sufficient G6PD activity can lead to that oxidation damaging and killing the red cell. And as you work your way down through that column, you will see that an adequate production of NADPH and glutathione leads to oxidation damage. Another enzyme disorder is pyruvate kinase deficiency. Again, a similar but rare disorder. Finally, the 3rd inherited course of hemolytic anemia, inherited hemoglobinopathies. 14:55 These are very common and we're now pretty sure that they've been selected during evolution because a heterozygous state, although it's not clinically insignificant in the patient, it provides protection against severe malaria. Malaria has had a big influence on the genetic make-up with people who live in malarial regions. But unfortunately homozygous forms a bad gene from your mom and your dad, can be very severe disorders. Let's look particularly at sickle cell disorders in this case. Again on the right, an electrophoretic analysis of globin. At the top we have a normal person, hemoglobin A dominating with a small amount of hemoglobin F. Next one down, sickle cell anemia, a mutation in the beta chain. So there's no normal hemoglobin A. What we have is hemoglobin S. I'll show you on the next slide what that does to red cells. The 3rd one down is sickle cell trait. This is the heterozygous state, 1 normal beta globin gene and 1 sickle beta globin gene. And here we see low levels of hemoglobin A and some hemoglobin S, that's largely asymptomatic. And at the bottom for the aficionados if hemoglobinopathies is someone who has a sickle beta gene and the hemoglobin C beta gene and you'll see now you get hemoglobin C, S, and no normal A. Sickle cell anemia is a very severe disease. In that slide, you'll see a classic sickle cell similar to the ___ which are used for cutting corn. Now sickle cell anemia leads to sickling of red cells during periods of hypoxia. When the blood is deep within capillaries and becomes hypoxic, the hemoglobin stacks up and leads to sickling of the cells. This can block the blood vessels and that can lead to a range of clinical problems largely due to infarction ___ and hypoxia of tissues further down. Treatment of sickle cell anemia, if needed, is with red cell transfusions to provide normal blood or with drug such as hydroxycarbamide which is shown to be quite effective. Finally in hemolytic anemia, let's consider those disorders which are required, they're not inherited but developed in life. And I think the major one I want to focus on is the autoimmune hemolytic anemias where the body makes antibodies against the red cells. Two types of antibodies are made, IgG antibodies which you will know are quite high affinity antibodies, they bind at 47 degrees and they're sometimes known as warm antibodies. Look at the top 2 pictures on the right there. On the left, you will see something we saw similar to what you saw a few slides ago, spherocytes. That's a characteristic feature of autoimmune hemolytic anemia and you'll notice more reticulocytes as well slightly bluish cells. On the right, you will see a stain for reticulocytes and that's because our body is responding due to the hemolytic anemia. The 2nd type of antibody that can be produced is IgM. These are less strongly binding. They often need lower temperatures to act, sometimes known as cold antibodies. But the very powerful at agglutinating red cells, look at the slide at the bottom of the picture. You see those clumps of red cells? They have been agglutinated by these IgM antibodies. These are sometimes found in older people who have plasma cells in the bone marrow producing these IgM antibodies and they can be triggered by infection as well.
About the Lecture
The lecture Types of Anemia – Red Cell Disorders by Paul Moss, PhD, OBE, FMed, FRCPath is from the course Hematologic Disorders.
Included Quiz Questions
Where does the body sequester iron in patients with chronic infections?
- Macrophages
- Neutrophils
- Lymphocytes
- Eosinophil
- Reticulocytes
Which of the following is given to a patient with chronic renal failure to stimulate red blood cell production?
- Erythropoietin
- Oral iron
- Parenteral iron
- Vitamin B12
- Folic acid
Which of the following does NOT cause a decrease in red blood cell production?
- Polycythemia Vera
- Leukemia
- Myeloproliferative disease
- Carcinoma infiltration
- Aplastic anemia
Which of the types of cell listed below is characteristically found in vitamin B12 deficiency?
- Megaloblasts
- Reticulocytes
- Metamyelocytes
- Myelocytes
- Promyelocytes
Which of the following statements accurately describes the pathogenesis of pernicious anemia?
- Auto-antibodies against gastric parietal cells
- Deficiency of intrinsic factor
- Pregnancy-induced auto antibodies
- Vitamin B12 deficiency
- Folate deficiency
Which of the following red blood cell parameters is increased due to the presence of reticulocytes in hemolytic anemia?
- Mean corpuscular volume
- Mean corpuscular hemoglobin concentration
- Mean corpuscular hemoglobin
- Hemoglobin
Below what approximate RBC lifespan can anemia be precipitated?
- 15 days
- 30 days
- 45 days
- 60 days
- 9 days
Which type of bilirubin will be increased in the blood of a patient with hemolytic anemia?
- Unconjugated and total bilirubin
- Conjugated and total bilirubin
- Unconjugated and conjugated bilirubin
- Delta bilirubin and total bilirubin
- Total bilirubin only
Which of the following molecules anchor the spectrin molecule?
- Band 3 protein, band 4.1 and Ankyrin
- Band 3 protein, band 4.1 and glycophorin A
- Band 3 protein, band 4.1 and glycophorin C
- Band 3 protein, band 4.1 and kinesin
- Band 3 protein, band 4.1 and a protein homodimer
Which of the following does NOT cause an oxidative stress that could precipitate a G6PD hemolytic crisis?
- Consumption of fruit
- Consumption of Fava beans
- Use of antimalarial medication
- Contracting a viral infection
- Necrotising enterocolitis
Which of the following describes the etiology of hemolytic crises in G6PD deficiency?
- Increased oxidative stress
- Spectrin deficiency
- Ankyrin deficiency
- Decreased globin production
- Substitution of a valine for glycine
Which of the following hemoglobinopathies is thought to convey some protection against malaria?
- Sickle cell anemia
- Pyruvate kinase deficiency
- Iron deficiency anemia
- Pernicious anemia
- Autoimmune hemolytic anemia
What is the temperature at which antibodies bind to red cells in warm autoimmune hemolytic anemia?
- 37 degree Celsius
- 37 degree Fahrenheit
- 4 degree Celsius
- 4-degree Fahrenheit
- 54 degree Celsius
Which of the following is the treatment of choice in patients with sickle cell anemia?
- Hydroxycarbamide
- Folic acid
- Hydrogen sulphide
- Vitamin B 12
- Iron supplementation
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
1 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |
Thank you Dr Paul for this practical and entertaining lesson