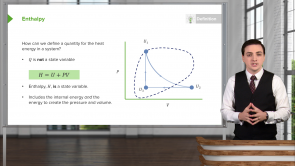
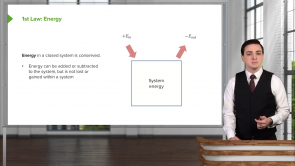
Standard Enthalpy of Formation

About the Lecture
The lecture Standard Enthalpy of Formation by Jared Rovny, PhD is from the course Thermodynamics and Thermochemistry.
Included Quiz Questions
Which of the following statements is FALSE?
- Endothermic reactions are usually hot.
- In a chemical reaction, if heat energy is taken in, the change in enthalpy is positive.
- If heat energy is released in a chemical reaction the change in enthalpy is negative.
- In breaking a compound substance, if heat energy is taken in, it is called an endothermic reaction.
- In breaking a compound substance, if heat energy is released, it is called an exothermic reaction.
In a chemistry lab, two compounds are mixed in a test tube, after which the test tube feels cooler. Which of the following statements is TRUE?
- The change of enthalpy of the system is positive.
- This is an exothermic chemical reaction.
- Heat energy is released in this process.
- The pressure of the system has reduced since the temperature has dropped.
- The change of enthalpy of the system is negative.
These courses may be of interest to you
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
5 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |