Pressure-Volume Diagrams: Cycle

About the Lecture
The lecture Pressure-Volume Diagrams: Cycle by Jared Rovny, PhD is from the course Thermodynamics and Thermochemistry.
Included Quiz Questions
Which of the following statements is FALSE?
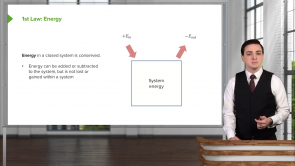
- In a cycle, if positive work is done by the system heat must be removed from the system in order to have ΔU = 0.
- In a cycle the system returns to its original pressure and volume.
- The total heat energy taken from the system is the area enclosed by the pressure-volume curve if the cycle direction is counter-clockwise.
- During a backward process in a cycle, positive work is done on the system.
- The net work done by the system in a cycle is positive if the cycle direction is clockwise and is given by the area enclosed by the pressure-volume curve.
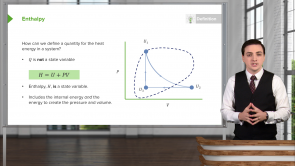
In a cycle process ΔU = 0. Which of the following is correct regarding the total heat transfer to the system Q and total work done by the system W in the cycle process?
- Q = W
- Q > W
- Q < W
- Q.W ≤ 0 and |Q| = |W|
- Q = W = 0
In a cycle process on a pressure-volume diagram what is the net work done by the system? Consider A to be the area enclosed by the cycle curve.
- If the cycle direction is clockwise A is the net work done by the system otherwise if the cycle direction is counter-clockwise -A is the net work done by the system.
- If the cycle direction is clockwise -A is the net work done by the system otherwise if the cycle direction is counter-clockwise A is the net work done by the system.
- The net work done by the system is the work done by the system when the volume of the system is expanding.
- The net work done by the system is A.
- The net work done by the system is the work done by the system when the volume of the system is expanding plus the work done on the system when the volume of the system is contracting.
These courses may be of interest to you
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
5 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |