Hess's Law

About the Lecture
The lecture Hess's Law by Jared Rovny, PhD is from the course Thermodynamics and Thermochemistry.
Included Quiz Questions
Which of the following is Hess's law?
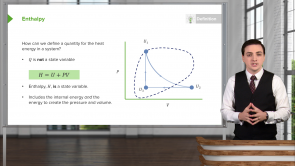
- The total enthalpy change in a reaction is independent of which steps or pathway is taken to go from the initial state to the final state.
- Zero of entropy is to take place at the absolute zero of temperature.
- If two systems are in thermal equilibrium with a third system, then these two systems are in thermal equilibrium with each other.
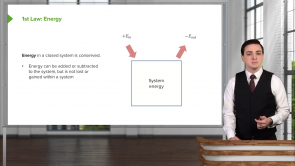
- Energy in a closed system is conserved.
- The entropy of a system will always increase.
These courses may be of interest to you
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
5 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |