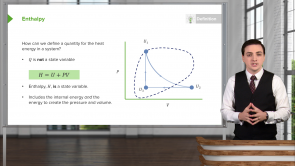
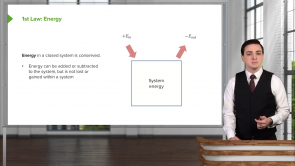
Heat of Phase Changes

About the Lecture
The lecture Heat of Phase Changes by Jared Rovny, PhD is from the course Thermodynamics and Thermochemistry.
Included Quiz Questions
Which of the following statements is FALSE?
- The heat of fusion per mol and the heat of vaporization per mol of a substance are the same
- The temperature of a substance is the same on either side of the phase change, but the energies are different
- At phase transition, heat is added without changing the temperature of the substance
- The heat required to melt a solid is the heat of fusion
- The heat required to vaporize a liquid is the heat of vaporization
How much heat is needed to vaporize n mol of a substance at its boiling point if its vaporization heat per mol is ΔHᵥ? Choose the best answer.
- nΔHᵥ
- Hᵥ
- nΔHᵥ + ncΔT
- 2nΔHᵥ
- ncΔT
Approximately how much heat is needed to turn 1 kg of 0°C ice into 1 kg of 100°C water vapor? (For water: ΔH(fusion) = 334 J/g, ΔH(vaporization) = 2260 J/g, c = 4.18 J/(g.K))
- 3,010 kJ
- 2,000 kJ
- 2,800 kJ
- 420 kJ
- 2,590 kJ
These courses may be of interest to you
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
5 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |