Emission and Absorption Spectrum

About the Lecture
The lecture Emission and Absorption Spectrum by Jared Rovny, PhD is from the course Electronic Structure.
Included Quiz Questions
Which of these is TRUE about an electron which moves from a higher energy level to a very close but lower energy level in hydrogen?
- It releases a red (or visible long wavelength) photon.
- It releases a blue (or visible short wavelength) photon.
- It always releases the same type of photon because all energy levels are discretely spaced.
- It releases a red (or visible short wavelength) photon.
- It releases a blue (or visible long wavelength) photon.
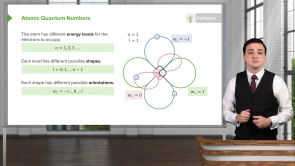
What information can be inferred from the emission spectrum of an atom?
- The distribution of electron energy levels
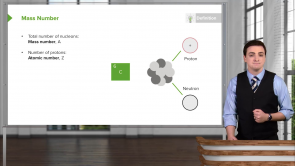
- The distribution of neutrons and proton energy levels in the nucleus
- The speed of the electrons orbiting the atom
- The distribution of energy among the particles in the nucleus
- The atomic mass number of the atom
What is the interesting relation between the absorption and emission spectrum? Choose the best answer.
- The missing wavelengths in the absorption spectrum are the same as the emitted wavelengths in the emission spectrum.
- In both a prism is used to split the light emerging from the material into its spectrum.
- In both the spectrum is revealed on a screen.
- In the absorption spectrum light is shined on the material but in the emission spectrum light is emitted from the material
- Both the absorption and emission spectrum can be used to obtain information regarding the nucleus structure of the atom.
These courses may be of interest to you
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
1 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |
Straight to the point explained well. I have had a time trying to study physics. this makes stuff way easier