Electron Structure Notation

About the Lecture
The lecture Electron Structure Notation by Jared Rovny, PhD is from the course Electronic Structure.
Included Quiz Questions
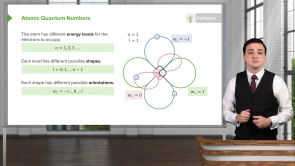
Which quantum numbers l do the letters d, f, p and s refer to?
- s : l = 0, p : l = 1, d : l = 2, f : l = 3
- d : l = 0, f : l = 1, p : l = 2, s : l = 3
- s : l = 0, p : l = 1, f : l = 2, d : l = 3
- s : l = 0, f : l = 1, d : l =2, p : l = 3
- p : l = 0, s : l = 1, d : l =2, f : l = 3
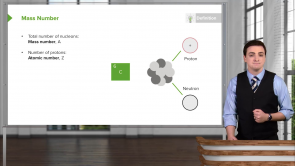
Which of the following choices is a possible electron configuration notation?
- 3p⁶
- 1p²
- 2d¹
- 1m⁴
- 0s²
How can the p orbital hold 6 electrons?
- Each of its 3 different orientations (m₁ = -1, 0, +1) can hold 2 electrons.
- The Pauli exclusion principle does not apply to higher orbitals.
- Both of the energy levels can hold 3 electrons.
- Its angular momentum quantum number l can range from 1 to 6.
- Each energy level can hold twice as many electrons as the prior level.
What is the electron configuration notation for when there is one electron in each of the possible orbital orientations for l = 2 and energy level n = 3?
- 3d⁵
- 3p²
- 3s¹⁰
- 3p⁵
- 3d¹⁰
These courses may be of interest to you
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
5 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |