Electron Configuration in the Periodic Table

About the Lecture
The lecture Electron Configuration in the Periodic Table by Jared Rovny, PhD is from the course Electronic Structure.
Included Quiz Questions
How many elements are in the “3p” section of the periodic table?
- 6
- 5
- 4
- 3
- 2
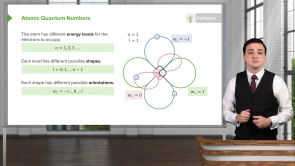
Why is the 3d section in the 4th row of the periodic table and not with the rest of the “3” row of the table?
- The periodic table is arranged in the order of added electrons, and electrons fill the “4s” section before the “3d” section.
- The “3d” section is put with its corresponding energy level because the electrons will fill lower values of “n” first always.
- The Pauli Exclusion Principle disallows electrons from filling the “3d” section before the “4s” section.
- This is just for historical reasons, and the “3d” section would be placed higher if the periodic table were recreated now.
- Electron configuration notation dictates that the energy level n = 4 comes before the angular momentum quantum number.
How is the electron configuration notation of elements in the periodic table commonly abbreviated?
- By using the previous noble gas symbol for the filled orbitals that match with the noble gas's filled orbitals and using electron configuration notation for the orbitals that are filled after.
- By referring each electron configuration from the prior angular momentum quantum number.
- By omitting the angular momentum quantum number when it can be easily deduced.
- By omitting the energy level quantum number when it can be easily deduced.
- By listing the energy levels independently of the other quantum numbers.
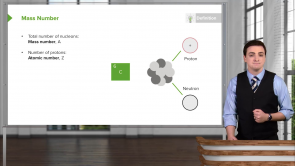
Potassium (K) is on the fourth row of the periodic table, one element after the noble gas Argon (Ar). What is its abbreviated electron configuration notation?
- [Ar]4s¹
- [Ar]3s⁴
- [Ar]4p¹
- [Ar]3s²4p⁴
- [Ar]5d¹
Why is Helium placed on the far right of the periodic table and not with the elements in the "S" columns?
- To keep with the other noble gases, which have their outer electron shells filled.
- Each row in the periodic table must span the full width.
- The Pauli Exclusion Principle disallows it from remaining in the s section.
- The n = 1 energy level can only hold one electron.
- The 1s section of the periodic table cannot have its outer shell filled.
These courses may be of interest to you
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
5 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |