Atomic Quantum Numbers

About the Lecture
The lecture Atomic Quantum Numbers by Jared Rovny, PhD is from the course Electronic Structure.
Included Quiz Questions
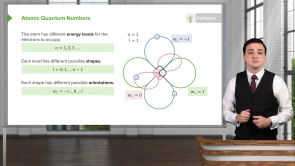
What are the four atomic quantum numbers and their possible values?
- n ( = 1, 2, ... ), l ( = 0, 1, ..., n-1 ), m_l ( = -l, ...,0, ..., +l ), m_s ( = ±1 )
- n ( = 1, 2, ... ), l ( = 1, ..., n ), m_l ( = -l, ...,0, ..., +l ), m_s ( = +1 )
- n ( = 1, 2, ... ), h ( = 0, 1, ..., n-1 ), m ( = 0, ..., +h ), p ( = ±1 )
- n ( = 1, 2, ... ), h ( = 0, 1, ..., n-1 ), m ( = 0, ..., +h ), p ( = -1 )
- n ( = 0, 1, 2, ... ), l ( = 0, 1, ..., n ), m_l ( = -l, ...,0, ..., +l ), m_s ( = ±1 )
How many different orientations can an electron's orbit with quantum number l have? What are the different values of the quantum number m_l corresponding to these orientations?
- 2l + 1, m_l = -l, ..., 0, ..., +l
- 2l, m_l = -l, ..., 0, ..., l -1
- l + 1 , m_l = 0, ..., l
- 2l - 1 , m_l = -l +1, ..., 0, ..., l -1
- 2l + 1, m_l = 0, ..., 2l
How many different shapes can an electron's orbit at energy level n have? What are the values of the quantum number l for these different shapes?
- n, l = 0, 1, ..., n-1
- 2n, l = 1, 2, ..., 2n
- 2n + 1, l = 1, 2, ..., 2n+1
- [n/2], l = 0, 1, ..., [n/2] -1
- n + 2, l = 0, 1, ..., n + 1
What does the Pauli exclusion principle tell us about electrons orbiting a nucleus?
- Two electrons cannot have the same 4 quantum numbers.
- Two electrons cannot have the same spin.
- Only one electron is allowed per energy level n.
- At energy level n, each orbit shape which corresponds to a quantum number l, can only hold one electron.
- The number of electrons and protons in an atom must be equal.
These courses may be of interest to you
Customer reviews
5,0 of 5 stars
| 5 Stars |
|
1 |
| 4 Stars |
|
0 |
| 3 Stars |
|
0 |
| 2 Stars |
|
0 |
| 1 Star |
|
0 |
I've taken Physics I and II before in college, and now am studying for the Mcat and really like how professor Jared breaks down these hard concepts into easy to understand morsels. Particularly in this lesson, he puts a new "spin" into quantum numbers leading to an easier explanation. Thanks!